This article covers
Microscopy Staining solution
Microscopy stains enhance the visualization of cells and cell parts under a light microscope. They give images more contrast and allow cells to be classified according to their shape (morphology). By using a variety of different stains, you can selectively stain different areas of the cells such as cell walls, nuclei, or the entire cell. Some types of stains can also distinguish living vs. dead cells.
Microscopy stains are salts composed of a positive and a negative ion, and one of which is colored. The colored positive ion called basic dye while the color in its negative ion is the acidic dye. Basic dyes such as Crystal Violet, Methylene Blue, Malachite Green, and Safranin are more commonly used than acidic dyes because bacteria and cell membranes are slightly negatively charged at pH7. The colored positive ion in the basic dye is attracted to the negatively charged bacteria cell wall and cell membranes. Therefore, we can see the outline of bacteria and cells easily after staining.
DNA is another example. DNA is negatively charged, so scientists and health professionals use Methylene Blue, a basic stain, to visualize the DNA.
Some type of microscopy stains work on fixed (non-living cells) and some stains can be used directly on the living cells (vital stains).
Methylene Blue
Formula: C16H18ClN3S
Synonym: Tetramethylthionine chloride
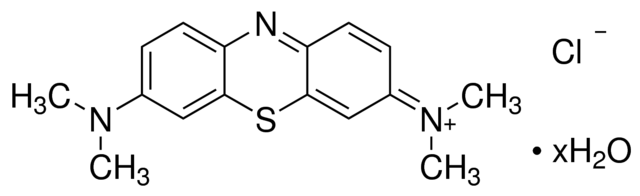
Methylene Blue is a popular basic (alkaline) stain that can be used to stain animal cells to make nuclei more visible.
Methylene Blue binds DNA, a very abundant material in the nucleus. Cells stained by Methylene Blue show the nucleus with a deep blue color. It also helps cells to show up against their background, where their shape can help you determine what they are (their morphology). You can substitute Methylene Blue in most staining protocols with carmine or Janus Green B. Methylene Blue is also used by aquarium hobbyists for preventing fungal disease.
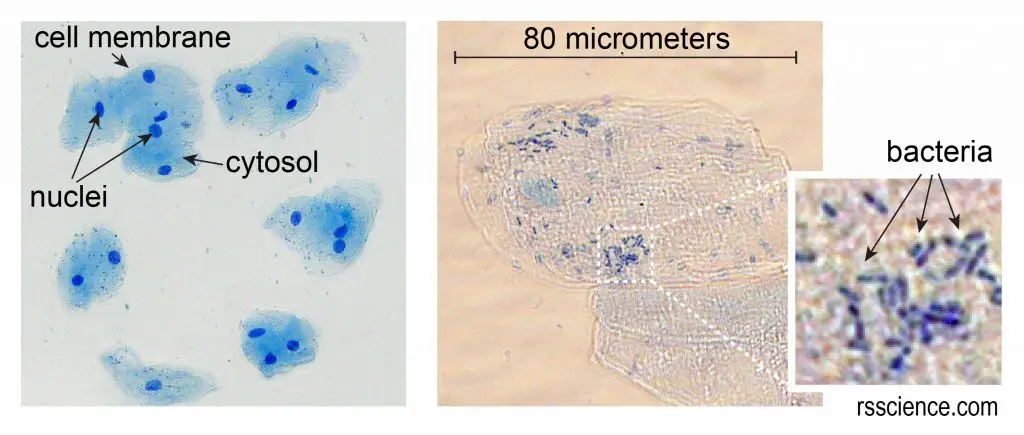
[In this figure] Cheek cells stained with Methylene Blue.
The left image is a low magnification. You can see the nuclei stained with a dark blue (because Methylene Blue stains DNA strongly). The cell membrane acts like a balloon and holds all the parts of a cell inside, such as a nucleus, cytosol, and organelles.
The right image is a high magnification. This cheek cell is about 80 micrometers in diameter. We measured the cell size by using a microscopic meter slide.
You can also see some small rod-shaped bacteria on the right image. Don’t worry, they are normal oral microbes.
You can purchase Methylene Blue staining solution (1% aqueous solution) here.
Eosin Y
Formula: C20H8Br4O5
Synonym: 2’,4’,5’,7’-Tetrabromofluorescein
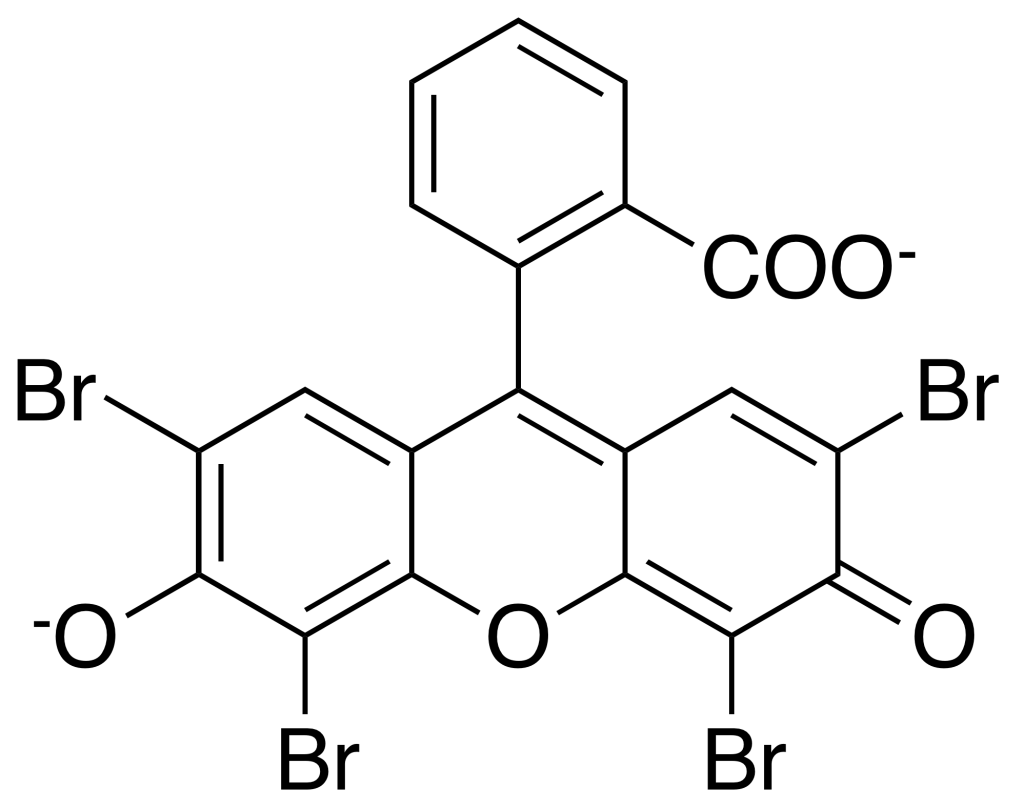
Eosin Y stain is a counterstain to Methylene Blue. It is most commonly used as an acidic red stain for highlighting colors of red blood cells, cytoplasmic material, cell membranes, and extracellular structures in pink or red colors.
In the hospital, Eosin’s most important medical uses are H&E (haematoxylin and eosin) staining. H&E staining is one of the most commonly used techniques in histology. Tissue stained with haematoxylin and eosin shows cytoplasm stained pink-orange and nuclei stained darkly, either blue or purple.
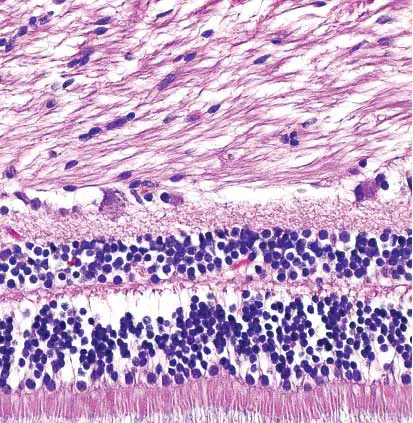
[In this figure] Retina stained with hematoxylin and eosin.
Cell nuclei stained blue-purple and extracellular material stained pink.
Photo credit, wiki.
You can purchase Eosin Y staining solution (0.5% aqueous solution) here.
Bismarck Brown Y
Formula: C18H18N8 · 2HCl
Synonym: 4,4′-(m-Phenylenebisazo)bis-m-phenylenediamine dihydrochloride; Vesuvine
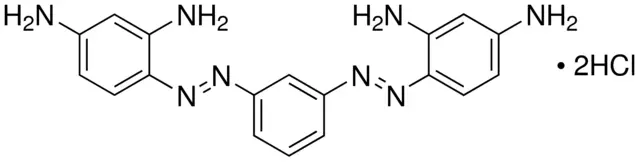
Bismarck brown Y is a common dye used in histology for staining tissues. Bismarck Brown Y (1% aqueous solution) is a light-brown solution. It stains acid mucins to yellow color. It is also used to stain cartilage in bone specimens and can be used with live cells (vital stain). Sometimes, in more advanced staining techniques, like Periodic Acid-Schiff (PAS) stain, Bismarck brown is used as a counterstain for the background.
Bismarck brown Y is certified for staining mucus in the goblet cells of the intestine. Goblet cells are columnar epithelial cells whose function is to secrete gel-forming mucins, which are the major components of mucus. Mucins are a family of high molecular weight, heavily glycosylated proteins produced by epithelial tissues. One of the key functions of mucins is their ability to form gels; therefore, they are a key component in most gel-like secretions.
Our body produces more than a liter of mucus every day and all the wet surfaces of your body, like your eyes, nose, mouth, lungs, and stomach, are coated and protected by mucus membranes. Mucus is also important for maintaining a healthy microorganism community and against infectious germs.
You can purchase Bismarck Brown Y staining solution (1% aqueous solution) here.
Janus Green B
Formula: C30H31ClN6
Synonym: 3-Diethylamino-7-(4-dimethylaminophenylazo)-5-phenylphenazinium chloride, Diazin Green S, Union Green B
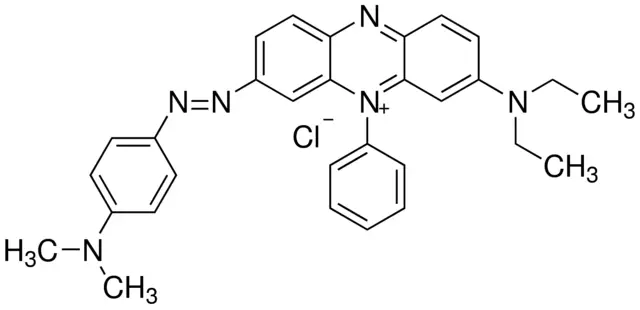
Janus Green B is a basic dye and useful vital stain. For example, Janus Green B can be used to observe the chloroplasts moving by cytoplasmic streaming in the cells of the aquatic plant Elodea (see the cool videos below). Janus Green B is also used to stain insect chordotonal organs (stretch receptors that can detect the position of the body) and peripheral nerves, where the nerves appear dark blue in comparison to non-stained tissue.
[In this video] Cytoplasmic streaming in plant cells (Elodea) – DIC / 1250x.
Janus Green B interacts with DNA and therefore, can be used to stain mitochondria, the powerhouses of the cells. Janus Green B can also function as an indicator that changes color according to the amount of oxygen present. When oxygen is present, the indicator oxidizes to a blue color. In the absence of oxygen, the indicator is reduced and changes to a pink color. In mitochondrial staining, Janus Green B can be an indicator to reveal the oxidation and reduction status of the electron transfer chain.
You can purchase Janus Green B staining solution (1% aqueous solution) here.
Neutral Red
Formula: C15H17ClN4
Synonym: 3-Amino-7-dimethylamino-2-methylphenazine hydrochloride; Toluylene red
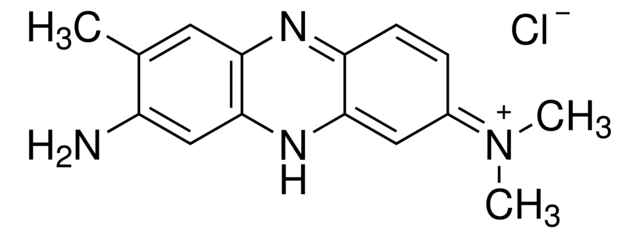
Neutral Red can be used as a vital stain to measure cell viability. Live cells incorporate Neutral Red into their lysosomes (an organelle in the cytoplasm of eukaryotic cells containing degradative enzymes). As cells begin to die, their ability to incorporate Neutral Red diminishes. Thus, loss of Neutral Red uptake corresponds to cell death. It is a common technique to evaluate virus infection in cell culture.
For histology, Neutral Red is used as a counterstain in combination with other dyes, and for many staining methods. For example, Neutral Red provides a pink background of cells to counterstain the dark brown staining of bone mineralization in von Kossa stain. For neurology, Neutral Red solution is excellent for visualizing neuronal cell bodies and the nuclei of non-neuronal cells in human brain sections.
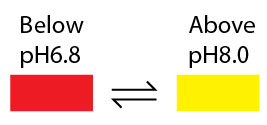
Neutral Red change its color from red to yellow between pH 6.8 and pH 8.0; therefore, is a pH indicator.
You can purchase Neutral Red Solution staining solution (0.33% in phosphate-buffered saline) here.
Gram’s Iodine Solution
Formula: 0.33% Iodine, 0.66% Potassium iodide in ddH2O
Gram’s iodine is used in Gram stain procedure to distinguish bacterial species into two large groups (gram-positive and gram-negative) based on the physical properties of their cell walls. The name comes from the Danish bacteriologist Hans Christian Gram, who developed this technique.
Gram stain distinguishes bacteria by the chemical and physical properties of their cell walls. Gram-positive bacteria contain a think layer of peptidoglycan, whereas Gram-negative bacteria only contain a thin layer of peptidoglycan. Because of this difference, the cell wall of Gram-negative is dissolved when the alcohol is present. This is why the cell loses its initial color from the primary stain.
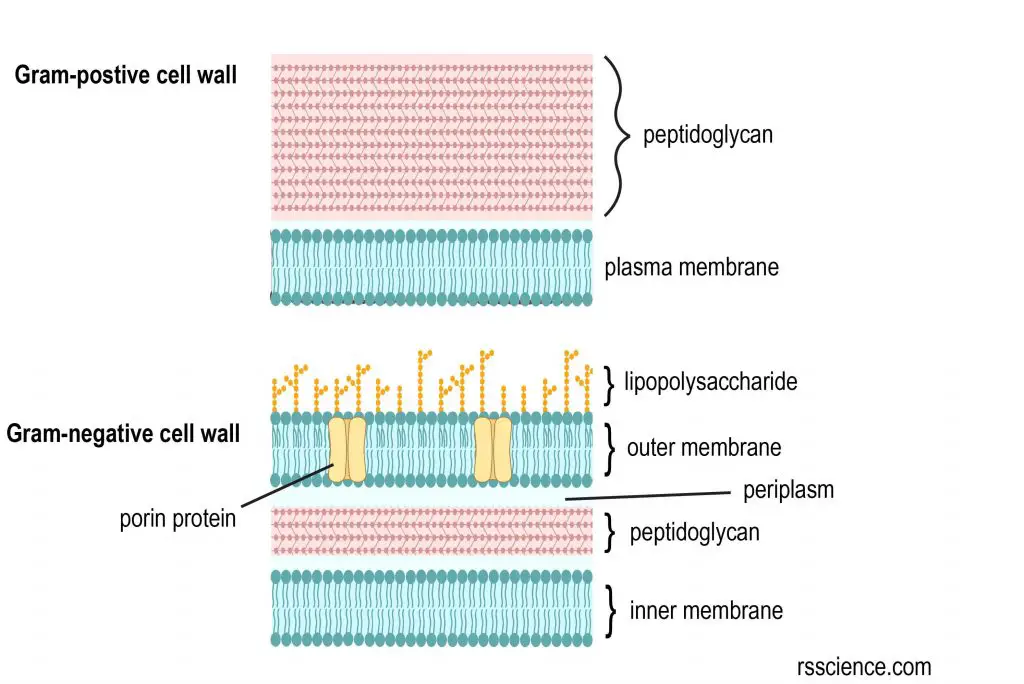
[In this figure] The cell wall structural difference between gram-positive and gram-negative bacteria.
Gram-positive bacteria retain the crystal violet dye, and thus are stained violet, while the Gram-negative bacteria do not; after washing, a counterstain is added (commonly safranin or fuchsine) that will stain these Gram-negative bacteria a pink color. Both Gram-positive bacteria and Gram-negative bacteria pick up the counterstain. The counterstain, however, is unseen on Gram-positive bacteria because of the darker crystal violet stain.
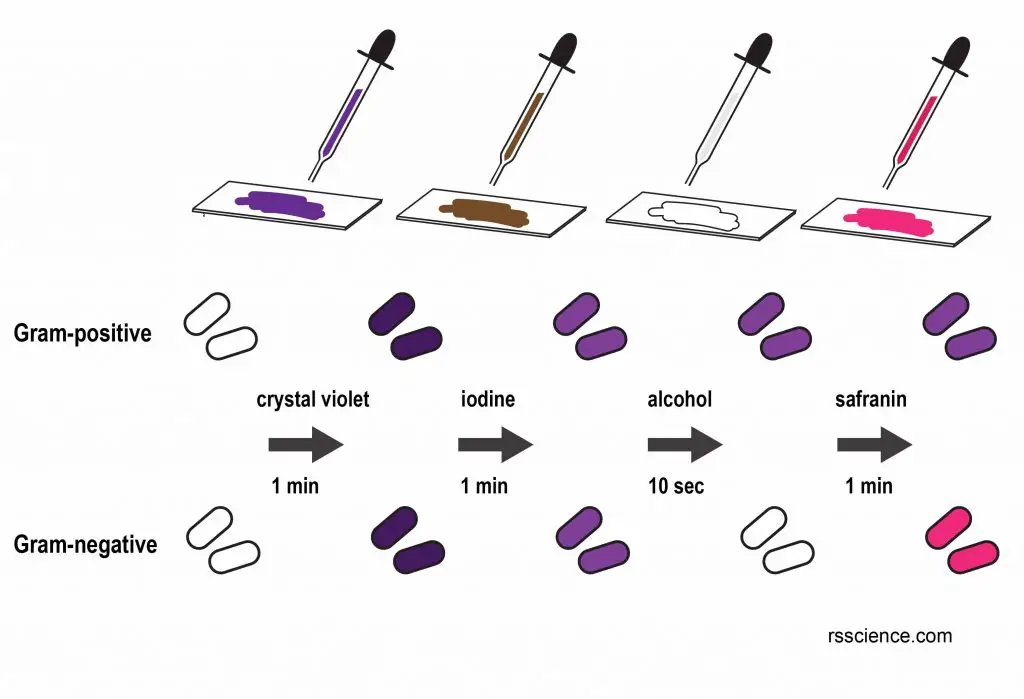
[In this figure] A procedure of Gram stain.
The Gram stain is always the first step in the preliminary identification of a bacterium. For medical applications, the Gram stain is performed on body fluid or biopsy when an infection is suspected. The Gram stain yield results much more quickly than bacteria culture and is particularly important when infection would make a significant difference in the patient’s treatment and prognosis.
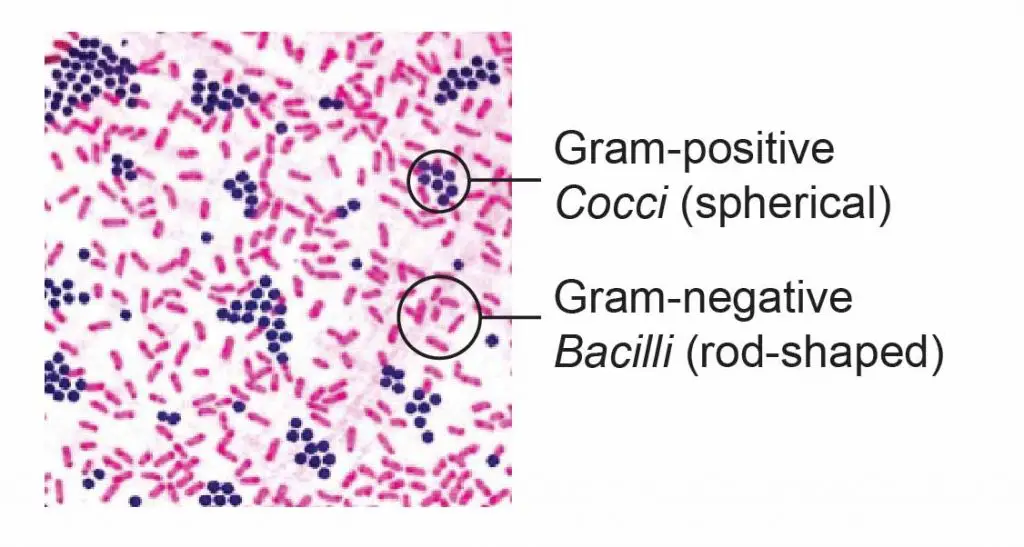
[In this figure] The gram-positive bacteria stain dark blue or purple while the gram-negative bacteria stain pink.
You can purchase Gram’s Iodine Solution staining solution (1% aqueous solution) here.
Copper(II) sulfate
Formula: CuSO4
Synonym: cupric sulfate, copper sulphate, blue vitriol
Copper(II) sulfate (CuSO4) is an inorganic compound with a bright blue color. It is the most common copper compound and has many uses in both industry and laboratory. Copper(II) sulfate can be used to kill algae and fungi, for example, treating aquarium fish for infections with very low concentrations.

[In this figure] Copper(II) sulfate
Photo source: Stephanb
It plays an important role in organic chemistry as catalyst and oxidizing agents. It is also used to detect sugars by turning soluble blue copper(II) into insoluble red copper(I) oxide when reduced by a sugar (Benedict’s solution).
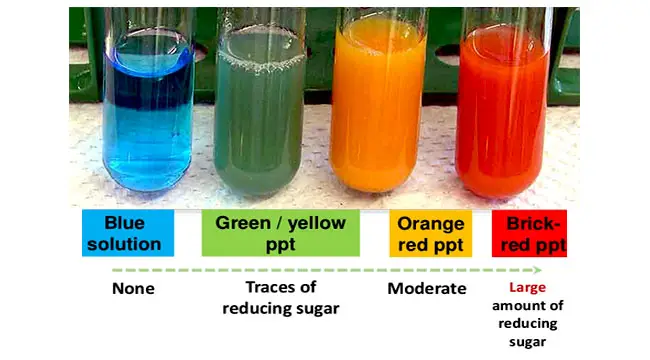
[In this figure] Benedict’s test result interpretation.
Photo source: microbiologyinfo
For medical applications, copper(II) sulfate is used to test blood for anemia. The test is done by dropping blood into a copper sulfate solution of known specific gravity. Normal blood will sink rapidly due to its density, whereas blood that does not sink or sinks slowly has an insufficient amount of hemoglobin.
Copper(II) sulfate can be used as a light blue counterstain for transparent objects, like human cheek cells or budding yeast, on microscope slides. It can also act as a chemical immobilizer when you’re observing live protozoa, such as paramecium. Copper ions can slow them done and keep them in the field of microscopic view.
High concentrated Copper(II) sulfate pentahydrate (some commercial Root Killer) is used to demonstrate crystallization. The blue crystals will form spontaneously during evaporation. It is possible to observe the growth of micro-crystals under a microscope.
You can purchase Copper(II) sulfate staining solution (1% aqueous solution) here.
Copper(II) acetate
Formula: Cu(CO2CH3)2
Synonym: cupric acetate
Copper(II) acetate is an inorganic compound with the chemical formula Cu(OAc)2 where OAc− is acetate(CH3CO2−). Anhydrous copper(II) acetate is a dark green crystalline solid, whereas monohydrous copper(II) acetate (Cu2(OAc)4(H2O)2) is more bluish-green. It has been used as a fungi killer and green pigments for a long time.
Today, copper(II) acetate are used as reagents for the synthesis of various inorganic and organic compounds. Copper(II) acetate is also a component of Barfoed reagent, which is widely used to detect the presence of monosaccharides (simple sugar) in an unknown solution.
For microscopy, the usage of copper(II) acetate is similar to copper(II) sulfate. It can enhance the contrast of transparent objects. It can also immobilize fast-swimming protozoa by exhausting them.

[In this figure] Cu(OAc)2 is a dark green crystalline solid.
Photo source: wiki
You can purchase Copper(II) acetate staining solution (3% aqueous solution) here.

