In this blog post, we will learn the natural habitation of paramecium; how to make paramecium culture at home for study; how to look at paramecia under a microscope; and their contribution to scientific discovery.
We also have 4 series blog posts about paramecium:
Part I. The Biological Classification of Paramecium – Name, History, and Evolution
Part II. The Structure of Paramecium cell
Part III. Paramecium Reproduction, Physiology, and Behaviors
This article covers
Where to find paramecia? – Their natural habitation
Paramecia are widespread in freshwater, brackish (slightly salty), and marine environments. They are often very abundant in the stagnant water of pools, lakes, ditches, ponds, and slow-flowing water that is rich in decaying organic matter.
Paramecia are attracted by acidic conditions since they feed on bacteria, which often slightly acidify their surroundings. They are an important link in the food web of aquatic ecosystems, feeding on bacteria. The dead organic matter often associated with these bacteria, which are preyed upon by other protists and small animals.
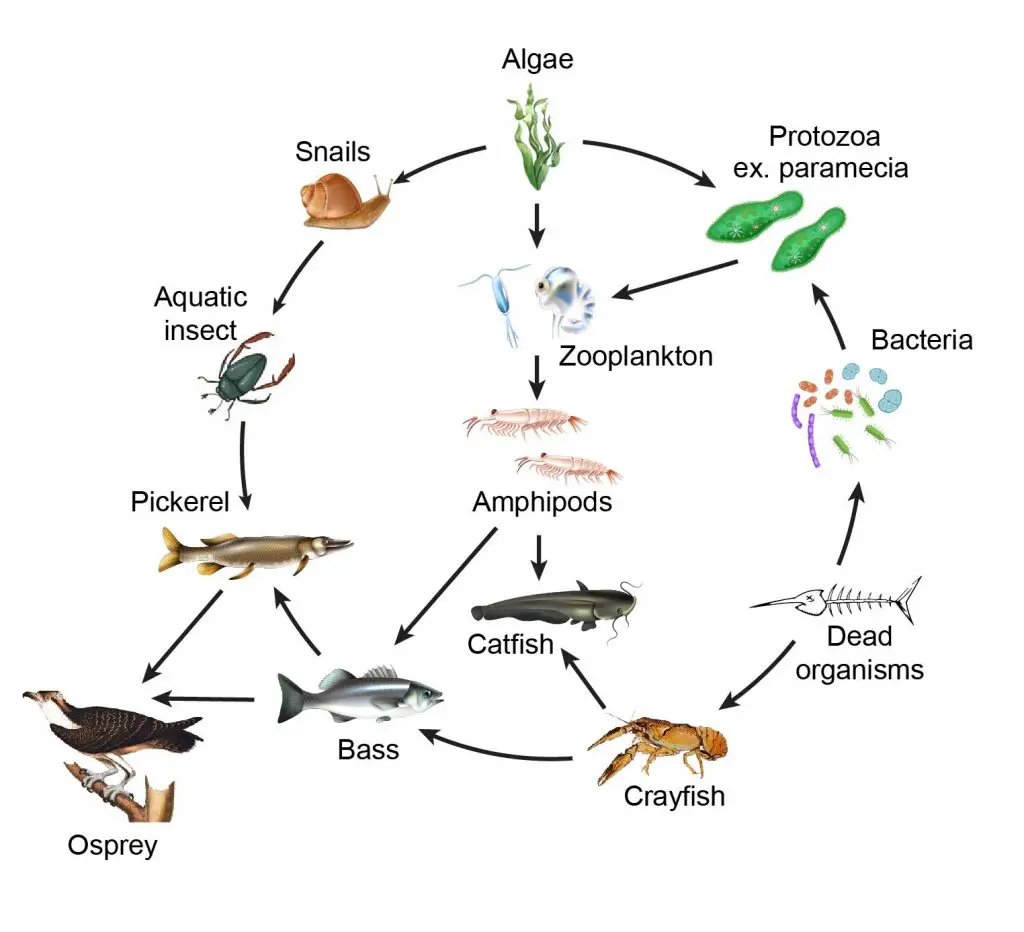
[In this figure] Food web of the aquatic ecosystem.
Every member of the ecosystem is essential. Protozoa like paramecia feed on bacteria and algae. Then, zooplankton and small crustaceans (for example, copepods and daphnia) prey for paramecia.
How to find paramecia for your microscopic project?
Paramecia are widespread in freshwater and are especially common in scums and ponds. You can use a transfer dropper to collect the water with bottom sediments to look for paramecia (and other microorganisms).
Where to collect the paramecia? Here are some pictures of habitations where I recently collect microorganisms for my microscopic project.

[In this figure] The places to collect microorganisms.
(A-C) Microorganisms stay with their source of food. Ponds or slow-flowing creeks with decayed organic materials in the bottom sediments (like leaves) are ideal habitations to find all kinds of microorganisms, like paramecia, amoebas, rotifers, water bears, daphnia, and diatoms. (D-E) I used the forceps to collect some decaying leaves and use a dropper to collect water with sediments into my sample vial. I will bring it home to look for microorganisms under my microscope.
How to culture paramecia in my laboratory, classroom or at home
Common paramecia are easily cultured in the laboratory without the need of special equipment. Because this, paramecia are widely used as model organisms to study many biological questions such as the competitive exclusion principle. Paramecia are also the most frequently studied microorganisms in middle school classrooms and science fairs.
You can grow paramecia at home in a small, low-budget scale.
Step 1 – Prepare the liquid food for paramecia
The “hay infusion” is perhaps the most well-known culture medium for microorganisms.
1. Boil one liter of the pond, spring or rainwater. Tap water should stand for 2-3 days to be dechlorinated.
2. As the water comes to a boil, add a small handful of hay (for example, timothy grass) and boil for ten additional minutes. The boiling will break down the hay to release the nutrients and set up an ideal medium for the growth of bacteria.
3. Allow this mixture to stand for two to three days.
4. Boling a few grains of uncooked rice or wheat, in the same way, could also be used as culture media.

[In this figure] Setting up a hay infusion for your microscope project.
Photo credit: Science lessons that rock
Different microorganisms have their favorite liquid food receipts. Check out Microbus’ website for more detail.
Step 2 – Initiate your paramecium seed culture
By handling newly collected specimens, I prefer to start with several small scales of cultures to test the optimal conditions.
1. I will prepare several 10 cm Petri dishes with 10-15 mL of hay infusion medium.
2. Add a few drops of water specimens into each dish. Ideally, in a few days, bacteria and small protozoans such as Chilomonas will populate your culture.
3. If paramecia are present, they will feed on these microorganisms and eventually increase in number.
4. Room temperature between 25-28oC without direct sunlight is suitable for paramecia to grow. You can place Petri dishes at several locations to see which one grows better.
5. This step may take 10 to 14 days, so be patient. Add new hay infusion medium if evaporation is strong.
6. You can observe the Petri dishes under a microscope to follow the condition of culture. If paramecia grow and become the dominate population in the dish, this can be maintained as your “seed culture”.
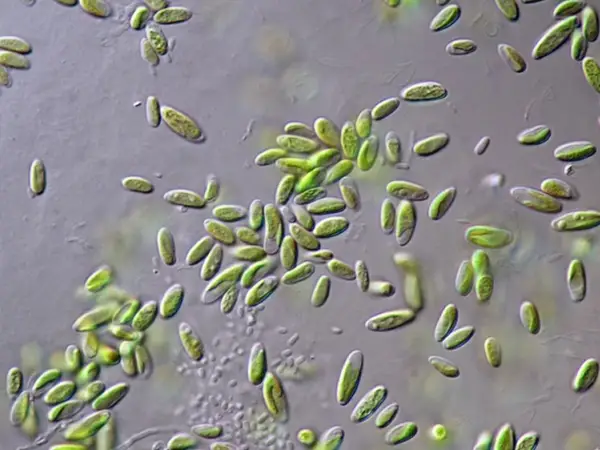
[In this figure] In an established seed culture of paramecia, paramecia should be the only dominant organisms.
Photo credit: ePond Shop
Established seed cultures can also be purchased from science supply companies like Carolina Biological Supply or Connecticut Valley Biological to save time.
Step 3 – Passage after a period of time to maintain your paramecia culture
To maintain your seed cultures, the waste needs to be removed and nutrients need to be replenished. We call this process as “passage” or “sub-culturing” of cell culture.
1. To do so, prepare a new petri dish with a fresh hay infusion medium.
2. Transfer a small portion of old liquid to the newly prepared dish (a process called inoculation). Transferring a smaller amount, or placing the Petri dishes at a cooler environment (18-20oC) could slow the growth of paramecia, which can reduce the burden of frequent sub-culturing.
For running a science classroom or laboratory, you may consider growing several small batches of seed cultures in other places as a backup in case of a rather rare “crash” in the paramecia population.
Step 4- Scale-up
Paramecia are nutritious food for baby fishes. Some aquarists may raise paramecia on a large scale to feed their fish tanks. This can be done by inoculating seed culture into a larger jar with liquid medium. You can use hay infusion or just add organic matter (like cut lettuce leaves) to feed the culture.
To accelerate the growth, keep the water warm in the 28oC by putting the jar in a windowsill with sunlight or shining a warm desk lamp on it. To minimize surface scum and horrible rotting smells, aerate the liquid by stirring it 2 to 3 times a day or adding a little air from an air pump.
The culture may first turn cloudy as the bacteria colony first grows. Then the culture will become clear and odorless as the paramecia eat up the bacteria. Always keep seed cultures in case the scale-up fails.

[In this figure] An example of paramecia cultured as a food for baby fishes.
A cut-off, clear, two-liter Pepsi bottle was used as the container. Lettice leaves were used as nutrient sources to grow bacteria. In 24 to 48 hours, the growth of bacteria makes the culture cloudy. Four days after the inoculation with paramecia, the culture turned clear after the paramecia eat up the bacteria.
Photo credit: Fishguy’s place
How to observe paramecia under a microscope
Paramecia are relatively large microorganisms (can grow up to 300 micrometers) with many visible granules inside the cells. Viewing live paramecia under a regular light microscope is not that difficult.
Use a transfer pipette and place a small drop of the specimen on a depression slide. Cover it with a coverslip and put it under the microscope. A regular microscopic slide may not have enough space; so, the paramecia will be squashed after placing the coverslip.
Start looking for paramecia at 10x. Once you get the images in focus, move up to 40x magnification. You may need to move the slide around a bit, but you should be able to locate living paramecia easily (if they are present in the specimen)!
[In this video] Paramecia and other small critters under the microscope.
From a freshly collected pond water, you may need patience to scan around for paramecia. But you may also encounter other microorganisms like amoeba and stentors as a bonus. If you are lucky, you may even see the interaction between microorganisms, like prey vs. predator. From a cultured specimen, you have a better chance to see the paramecia population in high density.
[In this video] An observation of amoeba eating paramecia under a microscope.
Paramecia swim happily by beating their cilia. Suddenly, some paramecia are captured by the pseudopods extended from an amoeba. These paramecia become a meal of the giant amoeba.
If you have an inverted microscope or high-power stereo microscope, you can observe the entire Petri dish by placing it on the specimen stage. It will be convenient to scan and search for your targets.
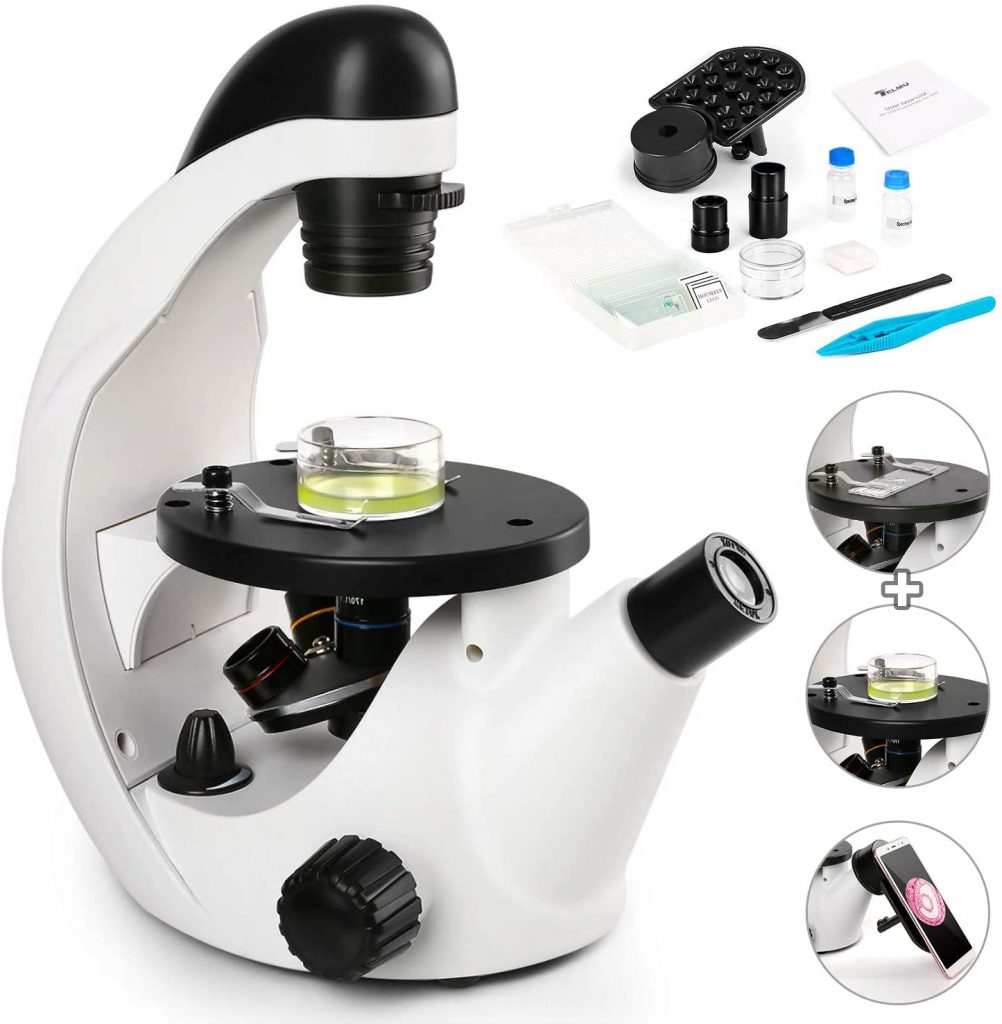
[In this figure] An example of an inverted microscope.
The inverted microscope is similar to the regular compound microscope but in an upside-down configuration. The objective lenses are now under the specimens. The advantage of an inverted microscope is that you can observe cells or microorganisms directly in the Petri dishes. But, remember that an inverted microscope is still a “transmission” light microscope (totally different from stereo microscopes). The specimens still need to be transparent and sitting close to the bottom of the Petri dish. Usually, objective lenses with a long working distance and phase contrast filters are equipped in professional inverted microscopes.
Image credit: Amazon
Premade slide set is a good alternative to look at paramecia
Living paramecia move consistently, make them difficult to observe for detailed cellular structures. You can add a small piece of cotton so that paramecia will be trapped in between the fibers to slow them down. Better resolution of paramecium’s internal organelles can be done by staining of fixed samples. Fixed paramecia can be preserved as permanent-mounted slides.
Color-stained paramecia specimen are included in Rs’ Science 25-slide sets. Other than paramecia, the slide set also includes microorganisms like spirogyra, penicillium, and daphnia, as well as specimens covering the biology of plant, animal, and the human body. Check it out!
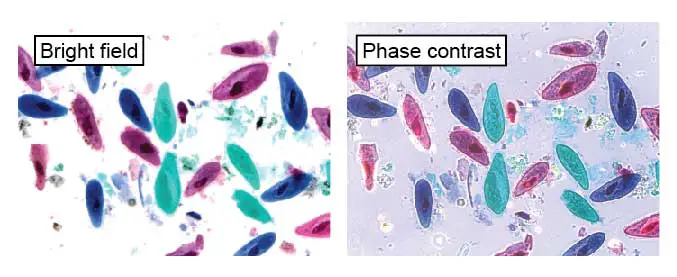
[In this figure] These paramecia were fixed and stained with colored dyes to make them visible.
The left image is a normal bright field image. The right image is through a phase contract filter to enhance the detail of cellular structures (like cilia). This slide can be found in Rs’ Science 25-slide sets.
Scientific discovery with the aid of paramecium – the competitive exclusion principle
Because paramecium is easy to grow and easily induced to reproduce and divide, it has been widely used in classrooms and laboratories to study biological processes.
Many important scientific discoveries are based on the study of paramecium. For example, Russian biologist Georgy Gause (1910-1986) proposed the Competitive Exclusion Principle, sometimes referred to as Gause’s law. This principle is based on experimental work done with mixed cultures of two paramecium species: P. aurelia and P. caudatum. This principle asserts that two species competing for the same limiting resource cannot coexist at constant population values. One will be slightly more efficient than the other and will reproduce at a higher rate as a result (in this experiment, P. aurelia reproduce faster than P. caudatum). The fate of the less efficient species is local extinction (in this case, P. caudatum).
This principle became fundamental to the science of ecology and population behavior. For example, paleoanthropologists speculate that the extinction of Homo neanderthalensis was due to its disadvantage in the competition with our ancestors (Homo sapiens).
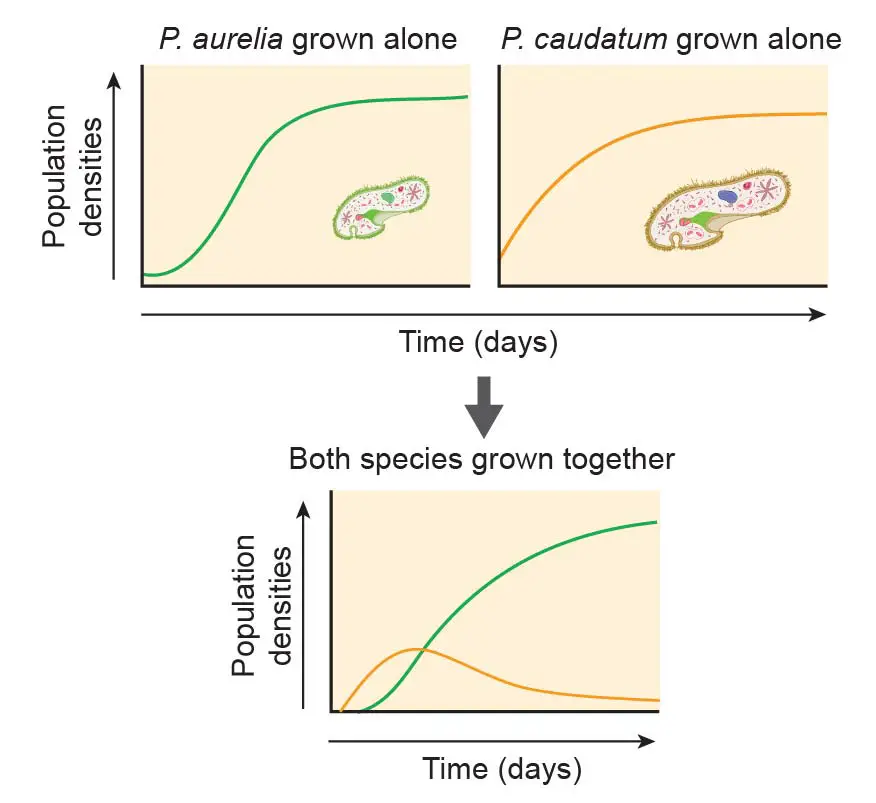
[In this figure] The demonstration of the competitive exclusion principle using two Paramecium species.
P. aurelia is the smaller but fast-growing species. P. caudatum is bigger but reproduce slowly. Both species grow happily in pure culture. However, once two species are mixed, even P. aurelia only has a slight reproductive advance, P. aurelia quickly compete and weed out P. caudatum.
Other discoveries which were done with paramecia (but have broad scientific meanings) mentioned in our previous contents are:
– Classification of Paramecium and the characteristics of Chromista kingdom
– The unique genome of Paramecium, its micronucleus and macronucleus
– Role of cytoplasmic streaming in single-celled organisms
– The life cycle of Paramecium and the significance of sexual production
– Special productive methods in Paramecium: autogamy, endomixis and cytogamy
– Paramecium and DNA damage theory of aging
– Paramecium as a model to study endosymbiotic relationship
– Chemical sensing in Paramecium
– Paramecium as a model to study learning and memory with no nervous system
– Biophotons as a way of cellular communication?
Predator and prey – the relationship of Didinium and Paramecium
Paramecium is also famous for their predator-prey relationship with Didinium, a genus of unicellular ciliates with a barrel-shaped cell body. The cell body is encircled by two ciliary bands that move Didinium through water by rotating the cell around its axis. When a Didinium finds a paramecium, it ejects poison darts (also called trichocysts) and attachment lines. The Didinium then proceeds to engulf its prey. Although paramecium is larger than Didinium, Didinium is a voracious eater and will be ready to hunt for another meal after only a few hours.
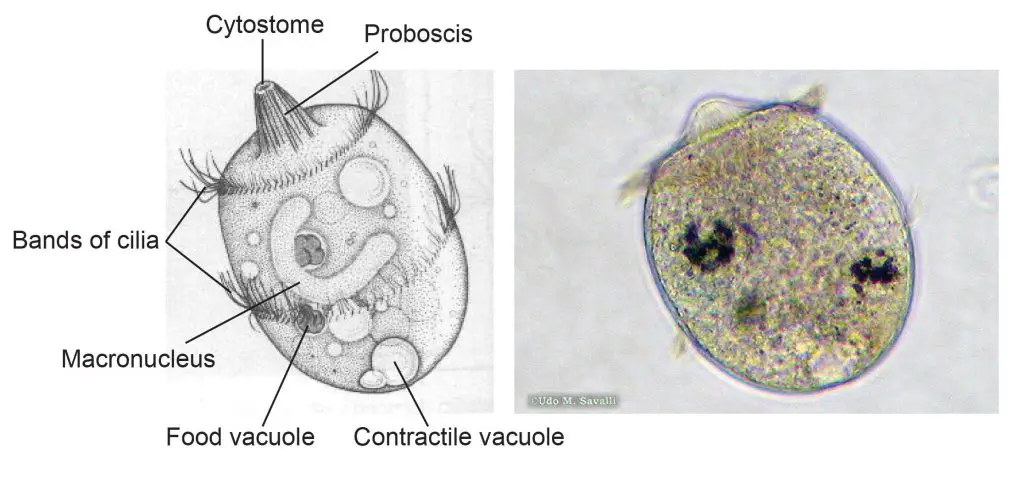
[In this figure] The structure of Didinium.
Left: a labeled diagram of Didinium. Didinium is also a ciliate, and so, you can find similar features that also be found in Paramecium. Right: a Didinium nasutum under a microscope.
Photo source: http://www.bcu.ubc.ca/courses/bio332/Labs/CiliateProject/didinium/DIDIweb.htm
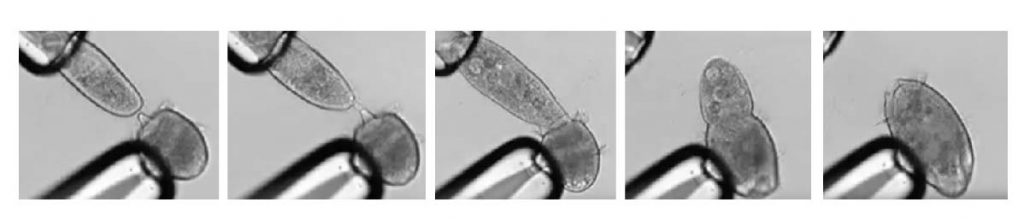
[In this figure] A series of screenshots shows the carnivorous ciliate Didinium nasutum capturing a prey, P. caudatum, and swallowing it.
Both Didinium and Paramecium were clamped by glass pipettes manipulated by scientists in order to measure their interaction.
Watch the original video by Schiegeli Acanthopagrus.
If you are interested in filming your own video, you can find Didinium with paramecium together in fresh and brackish water.

Pingback: Where does Paramecium live? – Dr. Biology Questions and Answers
Pingback: Is Paramecium autotroph or heterotroph? – Dr. Biology Questions and Answers
Pingback: Can the two Paramecium species prey on each other? – Dr. Biology Questions and Answers