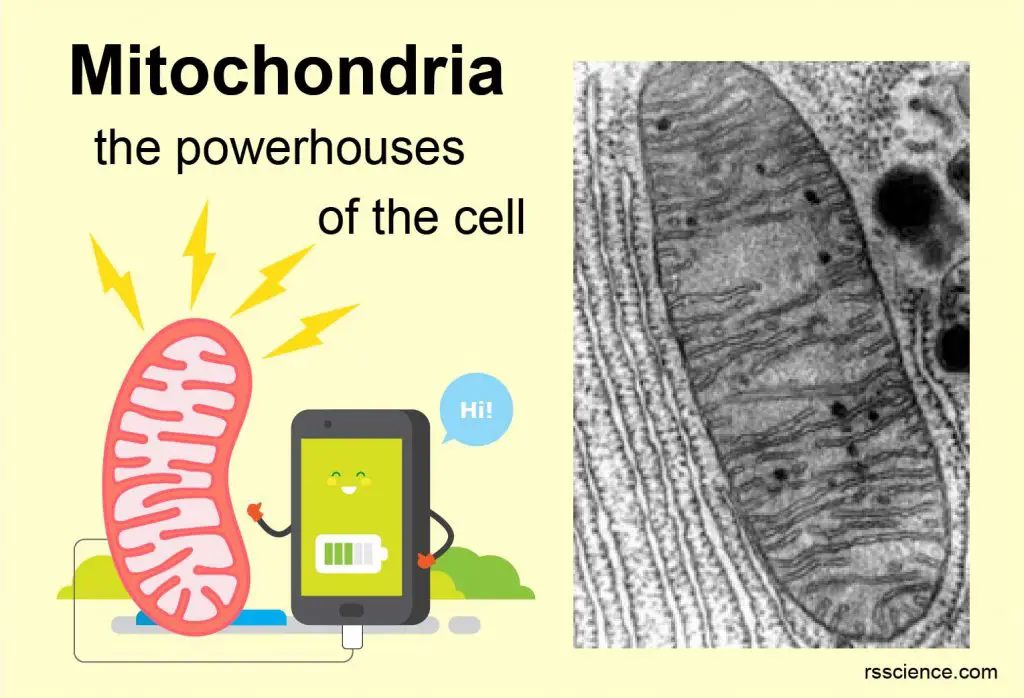
This article covers
What are mitochondria? A quick overview
Mitochondria (singular: mitochondrion) are rod-shaped organelles that are considered the power generators of the cell. During cellular respiration, mitochondria convert glucose and oxygen to produce adenosine triphosphate (ATP), which is the biochemical energy “currency” of the cell to do any cellular activities.
The numbers of mitochondria can reflect the energy demand of the cell type. For example, heart muscles host more mitochondria in order to power the heart pumping. On the other hand, our red blood cells lose their mitochondria as well as nuclei so that they can carry more oxygen.
[In this video] Golgi (green) and mitochondria (red) dynamics in fibroblast cells under a fluorescence microscope.
Two unique features of Mitochondria – double membranes and Mitochondrial DNA
Mitochondria have two unique features. First, mitochondria have two layers of membranes: the outer membrane and the inner membrane. These membranes divide a mitochondrion into compartments or regions, including intermembrane space, cristae, and matrix. This double-membraned structure is critical for the ATP generation. ATP is synthesized through the oxidative phosphorylation carried out by the electron transport chains across the inner membrane.
Second, Mitochondria (in plant cells, chloroplasts, too) are the only organelles that have their own DNA other than the nucleus. Mitochondrial DNA (mtDNA) is circular and encoded only 13 proteins.
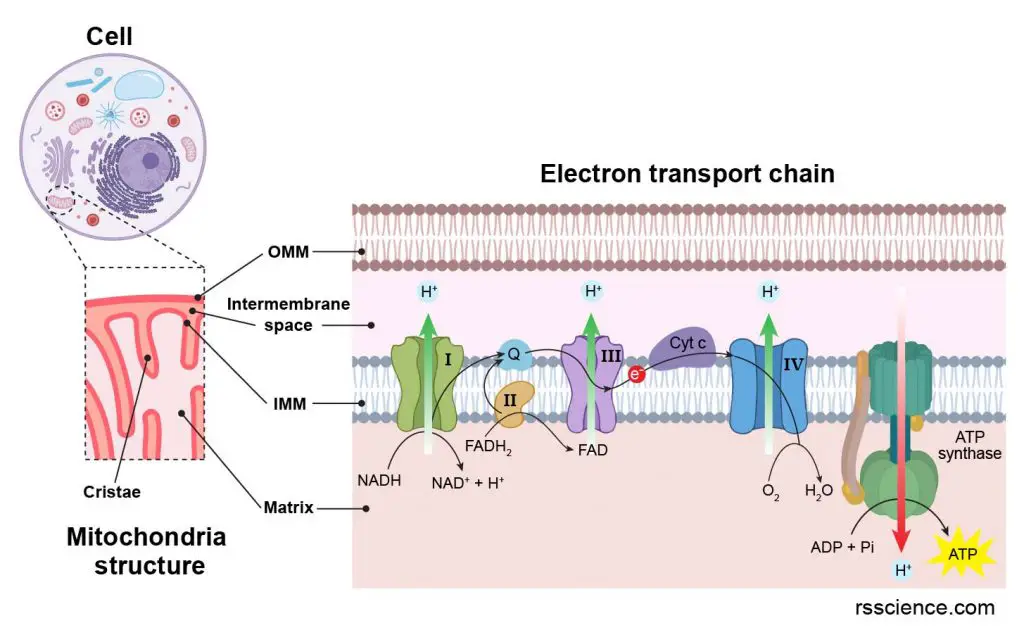
[In this figure] The mitochondrion structure closely relates to its ability to produce energy. The electron transport chains across the inner membrane are the major ATP generating sites in a mitochondrion.
The structure of mitochondria
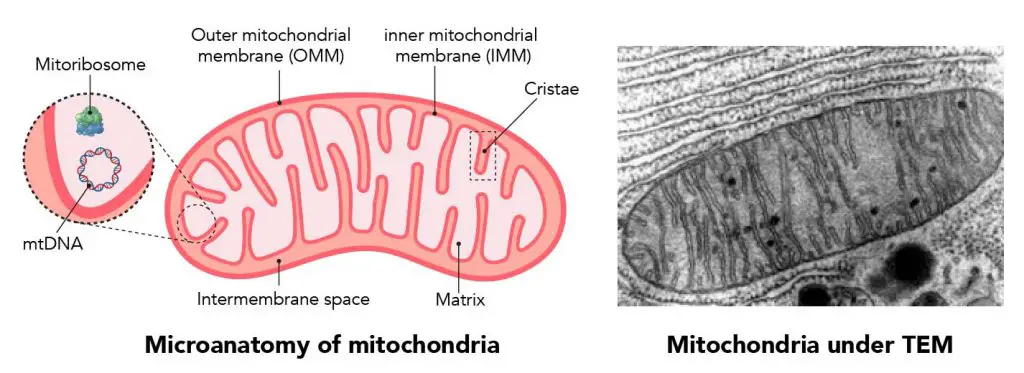
[In this figure] The basic diagram of a mitochondrion.
Left: the structure of mitochondrion showing many folds of membranes and mtDNA. Right: a mitochondrion surrounded by rough ER under a transmission electron microscope.
Mitochondria are small, often between 0.75 and 3 micrometers in size (about the sizes of bacteria), and are not visible under the microscope unless they are stained.
Unlike other organelles, mitochondria have two layers of membranes, an outer one and an inner one. Mitochondria are split into different compartments or regions by the membranes, each of which carries out distinct roles.
The major structures include:
Outer mitochondrial membrane (OMM)
Both mitochondrial membranes are made of phospholipid layers (same as the plasma membrane). The outer membrane covers the surface of the mitochondrion. This outer portion includes proteins called porins, which form channels that allow small molecules to pass. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion. Small molecules like nucleotides, ions, and metabolites can freely travel through the outer membrane. The outer membrane also hosts many enzymes with a wide variety of functions.
The OMM can associate with the endoplasmic reticulum (ER) membrane in a structure called MAM (mitochondria-associated ER-membrane). This is particularly important in the ER-mitochondria calcium signaling and is involved in the transfer of lipids between the ER and mitochondria.
Intermembrane space
Intermembrane space is also known as perimitochondrial space and is the area between the inner and outer membranes. Because the outer membrane is permeable to small molecules, the concentrations of small molecules, such as ions and sugars, in the intermembrane space is the same as in the cytosol. However, some proteins, such as cytochrome c, need special transporters across the outer membrane, so the protein composition in the intermembrane space is different from the cytosol.
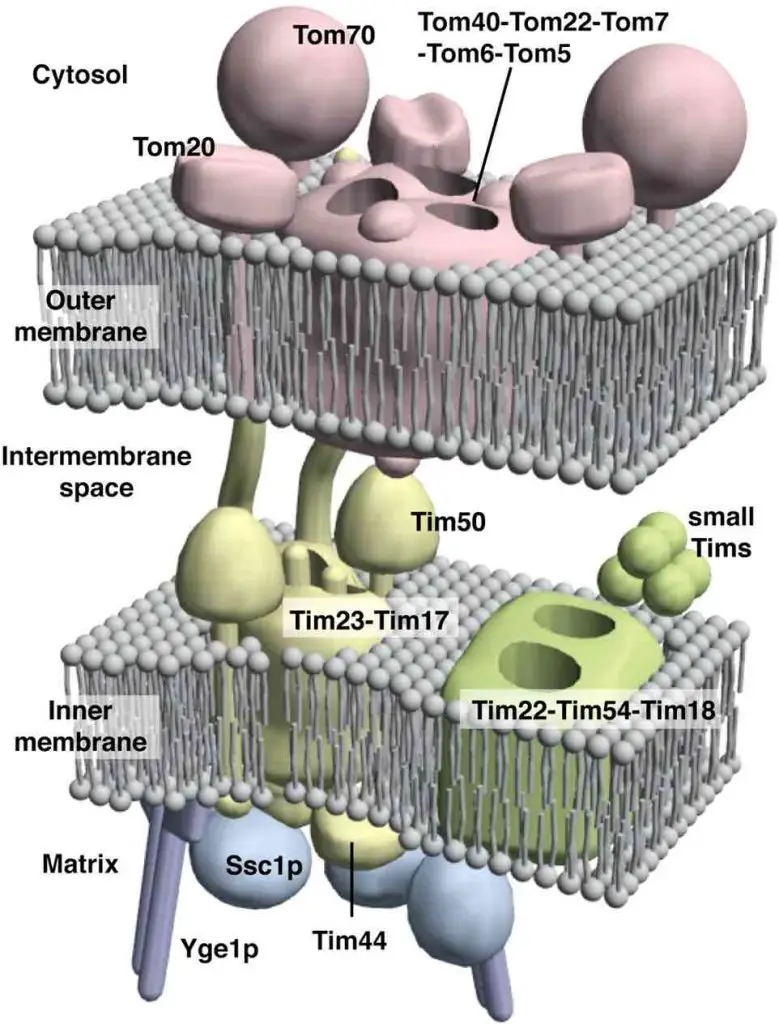
[In this figure] Sophisticated protein translocator systems across mitochondrial inner and outer membranes to deliver cytosol proteins (mitochondrial proteins but encoded in nuclear DNA) to the right compartments.
Photo credit: J of Cell Science.
Inner mitochondrial membrane (IMM)
The inner membrane is where most ATP is created. Because there are no porins in the inner membrane, it is impermeable to most molecules. This membrane holds proteins that perform the oxidative phosphorylation (OXPHOS) reaction. Basically, the proton (H+) gradient is built up across IMM by the electron transport chain. Then, ATP synthase cashes out the chemical potentials to generates ATP in the matrix. There are other specific transport proteins that regulate metabolite passage into and out of the mitochondrial matrix.
Cristae
Cristae are the folds of the inner membrane. They increase the surface area of the membrane, therefore increasing the space available for chemical reactions and enhancing its ability to produce more ATP.
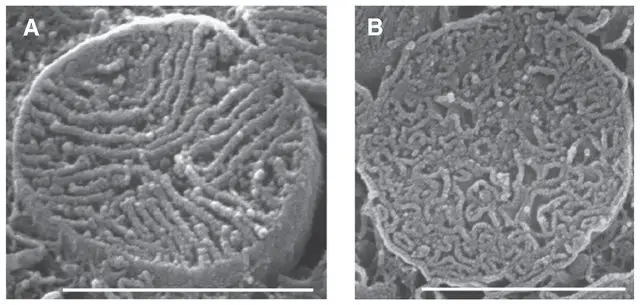
[In this figure] Scanning EM images showing the densely packed cristae in heart muscle cell’s mitochondria. Scale bars 1-μm; cristae width is about 30 nm.
Photo credit: Journal of Molecular and Cellular Cardiology.
Matrix
The matrix is the space enclosed by the inner membrane. Containing hundreds of enzymes, it is important for ATP production and many other biochemical reactions. Mitochondrial ribosomes, tRNAs, and several copies of the mitochondrial DNA genome is housed here.
Mitochondria function
Mitochondria produce energy and determine whether cells stay alive or commit suicide, a process called apoptosis. Mitochondria also regulate calcium concentration inside the cells and generate heat in the brown fat cells.
Mitochondria are the energy converters
The most prominent role of the mitochondrion is to produce ATP through cellular respiration. Mitochondria, using oxygen available within the cell to convert chemical energy from food to ATP, an energy form that is usable to the host cell.
What is ATP?
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive almost all processes in living cells. ATP is often referred to as the “molecular unit of currency” inside the cells. ATP is also a precursor to DNA and RNA.
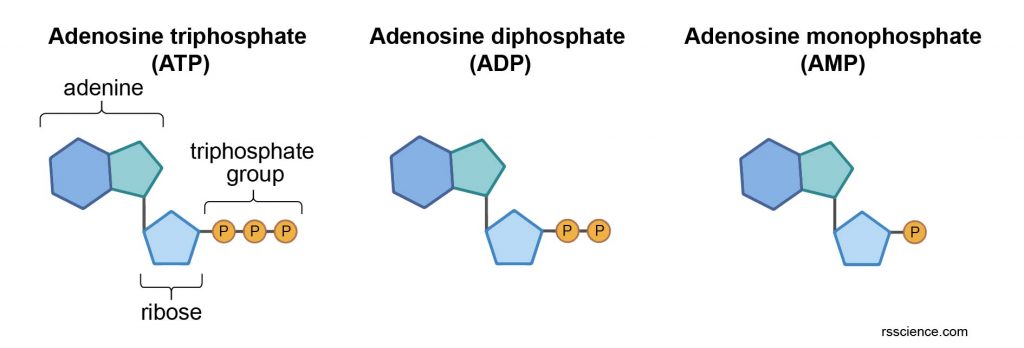
The energy is stored in the chemical bonds of phosphate groups. When consumed in metabolic processes, ATP converts either to ADP or to AMP.
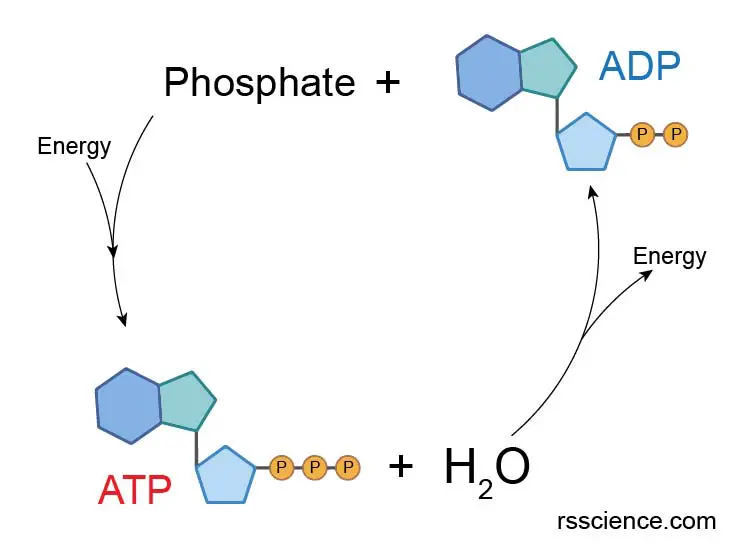
Mitochondria regenerate ATP by converting energy from food and oxygen, replenishing the energy pool of ATP reserve. The human body can recycle its own body weight equivalent in ATP each day.
Citric acid cycle in mitochondrial matrix
The biochemical reactions involved in ATP production are collectively termed as the citric acid cycle, also known as the TCA cycle (tricarboxylic acid cycle) or the Krebs cycle. The reaction happens in the matrix of mitochondria and produces a chemical called NADH.
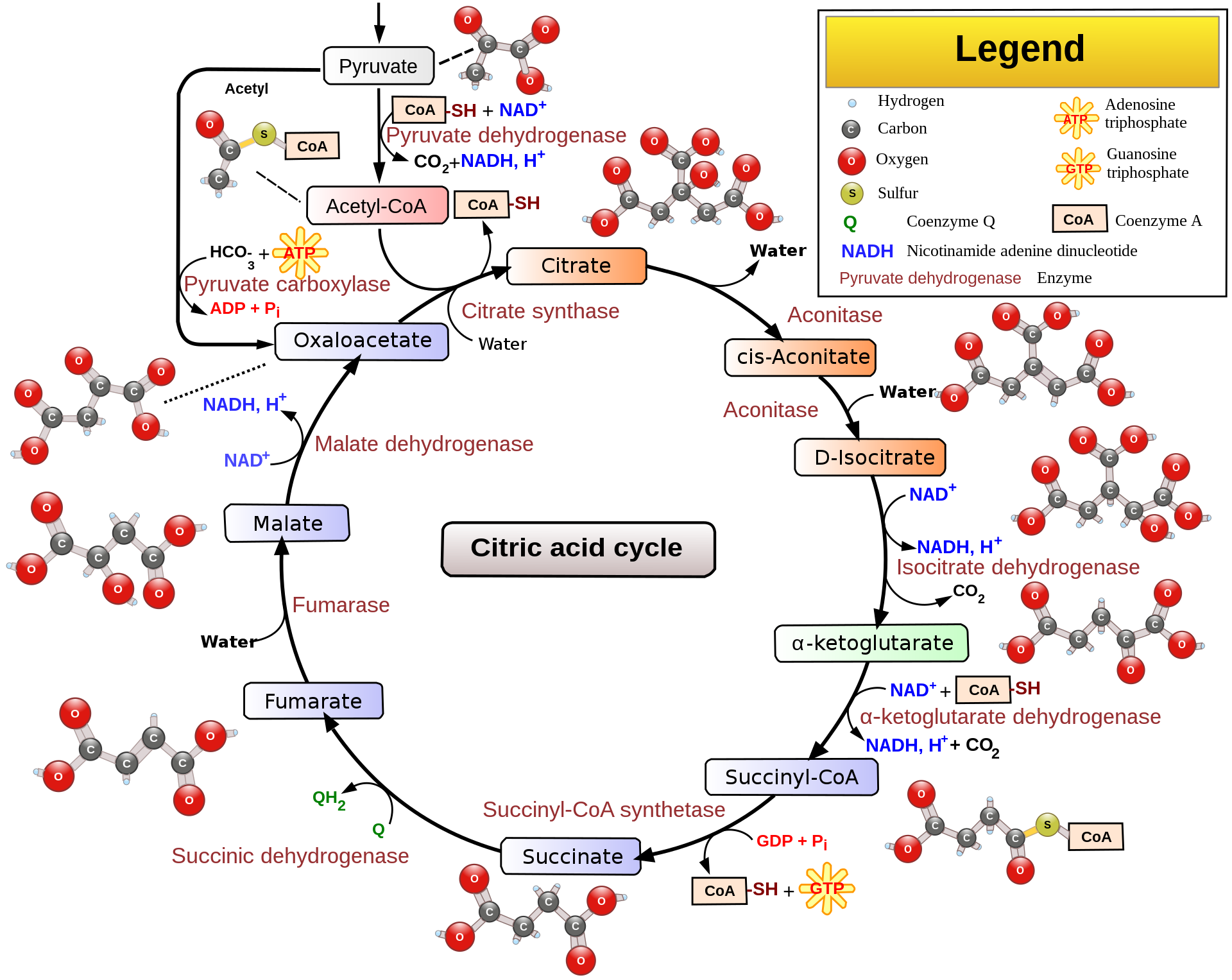
[In this figure] Overview of the citric acid cycle.
Pyruvate, the metabolism of glucose by glycolysis, is converted into acetyl-CoA, as the starting material of the citric acid cycle. Through a series of redox reactions, mitochondrial matrix harvests much of its energy in the form of NADH, FADH2, and ATP.
Photo credit: wiki.
For more details about the TCA cycle, see khan academy.
Oxidative phosphorylation and electron transport chain
The reduced electron carriers (NADH, FADH2) generated in the TCA cycle will pass their electrons into the electron transport chain (ETC), which will generate most of the ATP produced in cellular respiration.
:max_bytes(150000):strip_icc()/cellular_respiration-8fcc3f1ad3e54a828dabc02146ce4307.jpg)
[In this figure] Cellular respiration diagram.
One glucose molecule as the fuel can generate 36-38 ATP (glycolysis = 2; Krebs cycle = 2; OXPHOS = 32-34) through aerobic respiration (in the presence of oxygen), which is much efficient than anaerobic respiration. That being said, mitochondria are essential to all higher organisms for sustaining life.
Photo credit: Thoughtco.com
The double-layered structure is critical for the powerhouse function of mitochondria. In fact, the mitochondria generate ATP like a hydraulic dam. At the IMM, there is a set of proteins that assemble into an energy generator called electron transport chain.
In the first three steps of electron transport, these proteins pump the protons (H+; the hydrogen ion after losing its electron) from the matrix to intermembrane space (using energy stored in NADH and FADH2). Over time, this builds up a proton gradient across IMM. Then, at the last step, all the protons flow through a protein complex called ATP synthase, which acts as a turbine generator. ATP synthase uses the energy of proton flux to convert ADP into ATP. The process is called oxidative phosphorylation or OXPHOS.
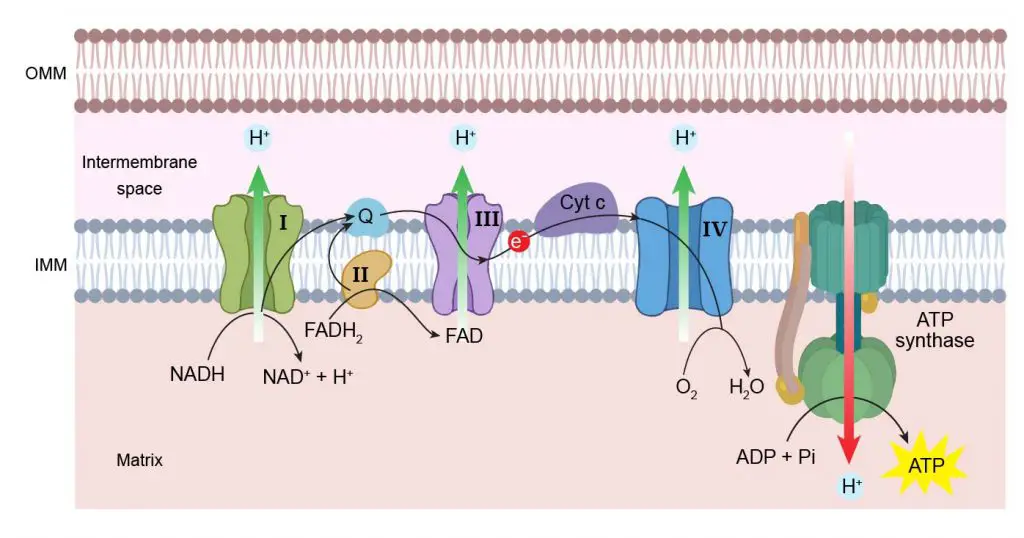
[In this figure] Electron transport chain.
The ATP generation happens on the inner mitochondrial membrane (IMM). First, protons (H+) are pumped across IMM into intermembrane space to build up a proton gradient. Then, the ATP synthase uses the energy of proton influx to drive the chemical conversion of ADP to high-energy ATP. During this process, two electrons (e-) are transferred between protein complexes. These protein complexes are called electron transport chain.
[In this video] Animation of electron transport chain.
Mitochondria determine cell live or dead – apoptosis
Recent research indicates that mitochondria play quite a large part in determining whether a cell stays alive or dies. Apoptosis is a form of programmed cell death, or “cellular suicide.” It is different from necrosis (the messy way), in which cells die due to injury. Apoptosis (the tidy way) is an orderly process in which the cell’s contents are packaged into small packets of membrane for “garbage collection” by immune cells. Apoptosis can selectively kill certain cells in a tissue but leave neighboring cells unaffected.
Apoptosis involves the death of a cell, but it benefits the organism as a whole. For instance, apoptosis intentionally removes cells during finger development, eliminates potentially cancerous and virus-infected cells, and maintains balance in the body.
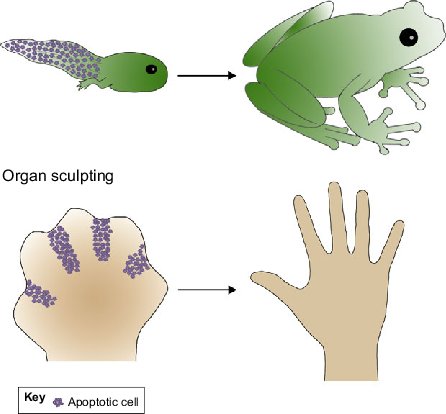
[In this figure] Roles for apoptosis in tissue and organ sculpting.
The tadpole tail is removed by apoptosis at the time of its metamorphosis into a frog. Apoptosis also selectively eliminates a certain portion of cells to sculpt the fingers.
Photo credit: Development.
Mitochondria play a key role in many apoptotic responses. Basically, mitochondria functions like a central control room, collecting different information (could be pro- or anti-apoptotic stimuli) in the cell and determining whether the cell should live or die. If the lethal signals pass a threshold, the OMM loses membrane potentials (an event called mitochondrial outer membrane permeabilization or MOMP); this leads to the release of various mitochondrial intermembrane space proteins (i.e., cytochrome c) into cytosol that activates caspases, resulting in apoptosis.
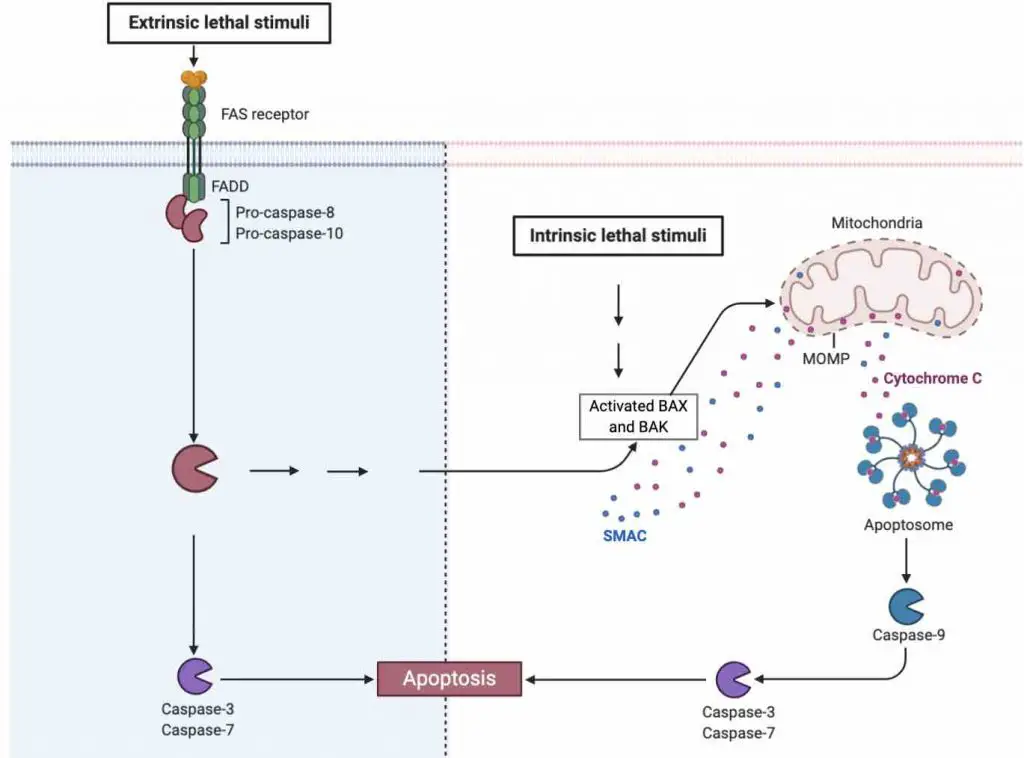
[In this figure] A schematic diagram showing the role of mitochondria in the apoptosis induced by extrinsic or intrinsic pathways.
Mitochondria produce heat
When we are cold, we shiver to keep warm (muscle movement generating heat). However, the body can also generate heat in other ways, one of which is by using a tissue called brown fat. This is known as non-shivering thermogenesis.
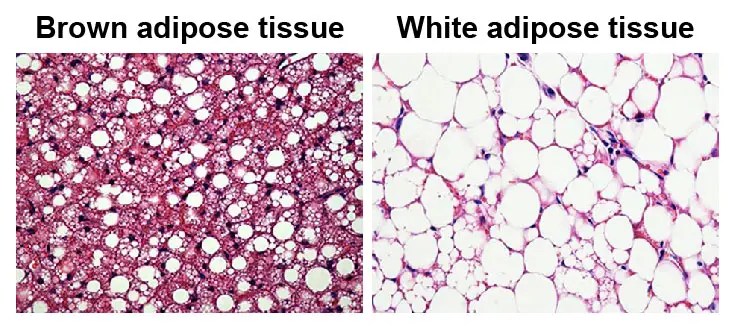
[In this figure] Histological images of brown adipose tissue (BAT) and white adipose tissue (WAT).
BAT gets a brownish color due to the high contents of mitochondria and capillaries.
Photo credit: Trends in Endocrinology & Metabolism.
Brown fat contains many more mitochondria than does white fat. However, these mitochondria are quite different. Instead of generating ATP by the end of the electron transfer chain, the protons “leak” in brown fat cells (brown adipocytes) by uncoupling proteins and generate heat. These mitochondria are the “engines” in brown fat that burn calories to produce heat.
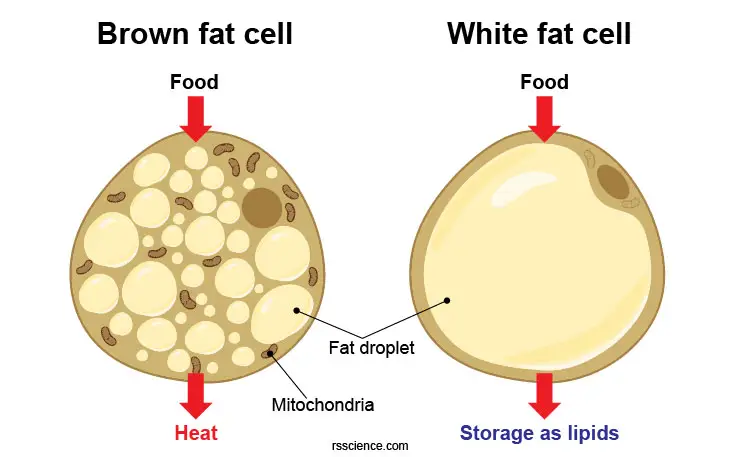
[In this figure] Brown and white adipocytes work quite differently although both are fat cells (contain fat droplets). White fat cells serve as energy storage for future needs. In contrast, brown fat cells burn fat storage to generate heat.
Brown fat is found at its highest levels in babies when we are more susceptible to cold, and slowly levels reduce as we grow up. However, people living in cold places were recently identified to have more brown fat tissues. Scientists believe that brown fat could be the key to the treatment of many metabolic diseases like type 2 diabetics.
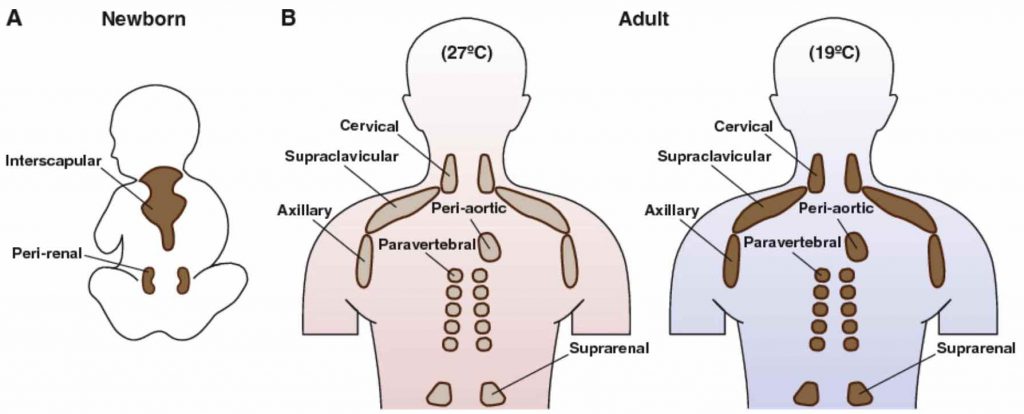
[In this figure] Brown fat locations in humans.
(a) Newborn infants have large interscapular and perirenal BAT depots. (b) In adults, smaller BAT depots are located primarily on the shoulder and back. Also, note that the amount of BAT is highly variable between individuals, but when active BAT is present, it has been shown to correlate with improved metabolism.
Photo credit: Handbook of experimental pharmacology.
Mitochondria store calcium
Calcium is a vital ion for many cellular processes; therefore, its concentration is tightly regulated. Mitochondria play a part in this by quickly absorbing calcium ions and holding them until they are needed.
Double-edged sword: Mitochondria generate ROS
During mitochondrial metabolism, reactive oxygen species (ROSs) or “free radicals” are generated as side products. ROSs can be used by cells as signaling molecules or weapons to kill bacteria. However, high dosages of ROSs can damage DNA, proteins, and lipids, too. Mitochondrial DNA is no exception. Since the mitochondrial DNA is located close to the energy converters, it can be heavily attacked; sometimes mutating ten times faster than nuclear DNA in an ordinary cell.
These mutations cause mitochondrial diseases that can affect high energy demand organs such as the heart, brain, muscles, central nervous system, and eye. People who have Parkinson’s or Alzheimer’s disease have a much higher mitochondrial mutation rate than healthy people, and the dysfunction of mitochondria may be implicated in these diseases. Mutations caused by ROSs have also been suggested as contributors to aging and cancer.
Mitochondrial DNA
Mitochondria are the only organelles that have their own DNA other than the nucleus (in plant cells, chloroplasts have their own DNA, too). Mitochondrial DNA (mtDNA) is circular (very similar to the bacterial DNA) and stored in the matrix. One mitochondrion can contain 2-10 copies of its DNA.
Compared to nuclear DNA, mitochondrial DNA is much shorter, encoding only 37 genes. These 37 genes produce 13 proteins for making the components of the electron transport chain, and 2 rRNAs and 22 tRNAs unique for mitochondria. The mitochondrial genome is only a small portion of the DNA in a eukaryotic cell; most of the DNA is in the cell nucleus (nuclear DNA).
Note: In 2001, a 14th mtDNA-encoded protein called humanin was discovered. This short peptide was shown to have neuroprotective and cytoprotective effects.
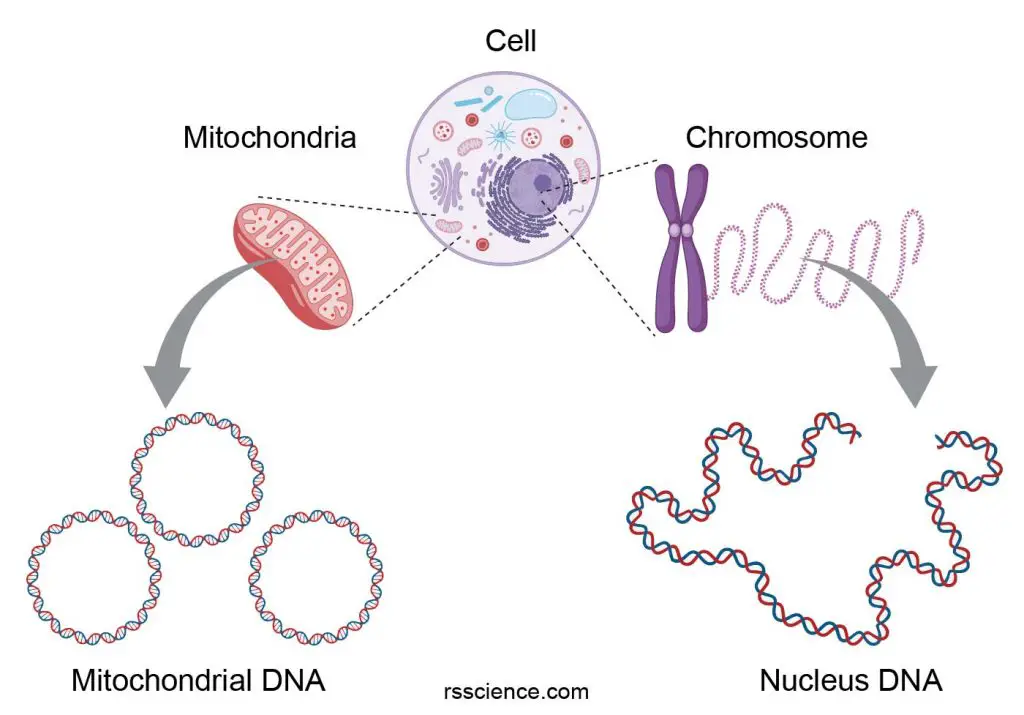
[In this figure] Animal cells have two sets of genomes: one from the nucleus and the other from the mitochondrion.
Mitochondrial DNA encodes two rRNA (12S and 16S) for the large and small subunits of mitochondrial ribosomes (mitoribosomes). mtDNA also contains 22 tRNA. Other than these, all other mitochondrial proteins (estimated 1,500 kinds of different proteins) are contributed by nuclear DNA, including DNA and RNA polymerase for the mtDNA replication and transcription.

[In this figure] The map of human mitochondrial DNA with the 37 coding regions (13 proteins, 2 rRNAs, 22 tRNAs) on their respective H- and L-strands.
In humans, mitochondrial DNA (mtDNA) forms closed circular molecules that contain 16,569 DNA base pairs.
Photo credit: wiki.
Mitochondria, and therefore mitochondrial DNA, are inherited only from the mother. This is because the sperm only provides its nuclear DNA, while all other organelles, including mitochondria, come from the egg. Any mitochondrial DNA contributed by the father is actively destroyed after a sperm fuses with an egg. Mitochondrial inheritance is non-Mendelian, as Mendelian inheritance presumes that half the genetic material of a fertilized egg derives from each parent.
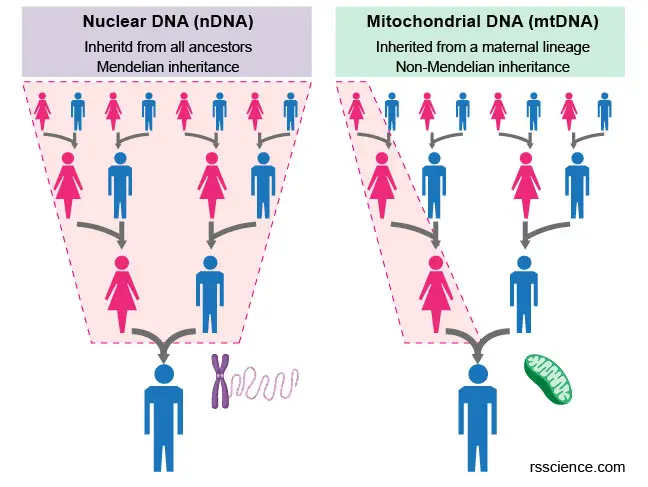
[In this figure] The comparison of nuclear and mitochondrial inheritance.
| Mitochondrial DNA | Nucleus DNA | |
| Location | mitocondrial matrix | nucleus |
| Size (human) | 16,569 bases | 3,300,000,000 bases |
| Genes (human) | 37 genes that encode 13 proteins, 22 tRNAs, and 2 rRNAs | approximately 30,000 genes |
| DAN form | small, circular | long, linear, arranged in chromosomes |
| Inheritance | inherited from the mother | inherited from both parents |
| Codon | mitochondrial codon | universal codon |
| DNA repair | no recombination | via recombination |
| Mutation rate | higher mutation rate | low mutation rate |
| Copies per cell | high copy number (500-2000) | two copies (2n) |
| Non-coding DNA | ~ 3% | ~ 93% |
| Intron | absent | present |
| Associate protein | largely protein free | histone or non-histone |
Mitochondria DNA genotyping provides our genetic history
All of us have mitochondrial DNA (mtDNA). Since your mitochondrial DNA is inherited from your mother – and she received it from her mother, and so on – it can shed light on the ancient origins of your maternal ancestors.
The DNA repair is poor in mtDNA so that mtDNA can accumulate unharmful mutations (or single nucleotide polymorphism, SNP; in mtDNA’s variable regions) much faster than nuclear DNA. Within a few thousand years, these mutations acquired in mtDNA are sufficient to distinguish an individual from different human populations. Mitochondrial haplogroups (also known as a maternal haplogroup) are families of mitochondrial DNA types that all trace back to a single mutation at a specific place and time. By sequencing our mtDNA, companies like 23andMe can learn how our ancient female ancestors migrated throughout the world by the geographic distribution of mtDNA types.
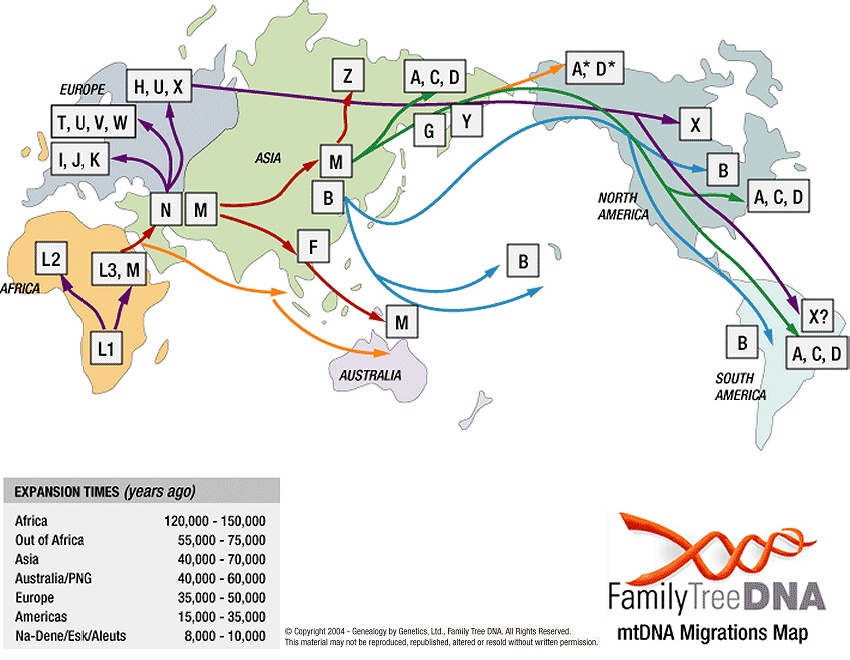
[In this figure] Mitochondrial DNA haplogroups migration map.
This genetic evidence supports the idea that the main pool of our ancestors came out of Africa about 200,000 years ago and that we did not descend from Neanderthals. Our mitochondrial DNA has descended from a common ancestral group of “Mitochondrial Eves” or “African Eves”.
Photo source: TranspacificProject.com
Mitochondrial DNA diseases
Mitochondrial diseases are a group of disorders caused by dysfunctional mitochondria. Mitochondrial disorders may be caused by mutations (acquired or inherited) in mitochondrial DNA or nuclear genes coded for mitochondrial components. They may also be the result of drugs, infections, or other environmental causes.
As mtDNA is copied when mitochondria proliferate, they can accumulate random mutations, a phenomenon called heteroplasmy. If only a few of the mtDNA copies inherited from the mother are defective, other normal copies can compensate, and the person is still healthy. The mitochondrial disease may become clinically apparent once the number of affected mitochondria reaches a certain level; this phenomenon is called “threshold expression.”
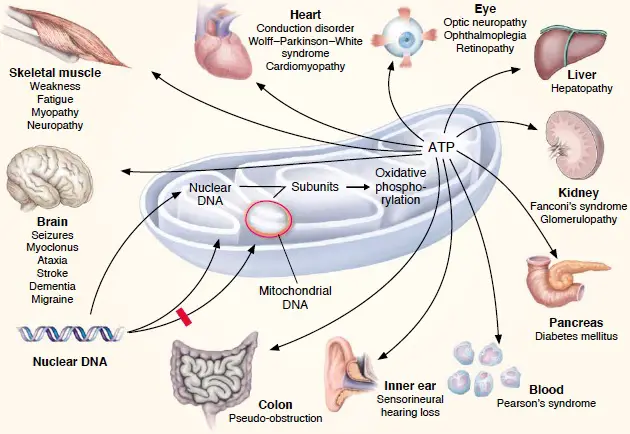
[In this figure] Mitochondria-related diseases.
Since mitochondria are so vital, anything that goes wrong in mitochondrial function can cause diseases. For example, several metabolic and neurodegenerative disorders affecting important body organs are linked to mtDNA mutations.
Photo credit: Donald R. Johns, MD.
The life cycle of mitochondria
Mitochondria are like tiny cells living inside another cell. They have their own life cycle. New mitochondria are generated by mitochondrial biogenesis, and old mitochondria are destroyed by mitophagy.
All mitochondria inside a cell is a pool
All mitochondria in a single cell are closely related. Instead of being individual rod-shaped organelles, mitochondria behave more like constantly changing tubular networks. Mitochondria can join the network by “fusion” and separate from it by “fission”. As a result, mitochondria (as well as their mtDNA and protein contents) are mixed as a pool.
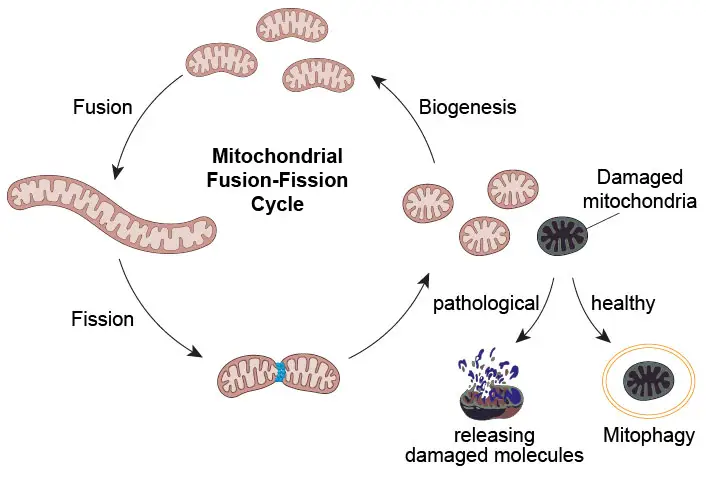
[In this figure] Fusion-fission cycle of mitochondria.
New mitochondria are born by biogenesis
When the cells receiving cues to increase their mitochondrial mass, they replicate their mtDNA and produce more mitochondria-related proteins (transcribed by both mtDNA and nuclear DNA). Proteins produced by nuclear DNA will be imported by special transporters to their destinations (OMM, intermembrane space, IMM, or matrix). This mitochondrial biogenesis enlarges the mitochondrial network. The short mitochondria are divided from the network by fission.
Fusion and fission cycle ensure mitochondrial quality
The processes of fission and fusion oppose each other and allow the mitochondrial network to remodel itself constantly. Damaged mitochondrial contents (mtDNA, proteins, and lipids) will be sorted out and separated from the healthy pool by fission. These damaged mitochondria will be eliminated by mitophagy.
[In this video] Time-lapse STED imaging of the inter-mitochondrial fusion.
Source: Wang C., et al., PNAS.
Mitophagy recycles damaged mitochondria
“Autophagy” (aka “self-eating”) is a biological process when cells are short of nutrient supply. To obtain the nutrient, the cell has to recycle some of its existing proteins and organelles, especially the misfunctioned ones. The autophagy to degrade bad mitochondria is named “mitophagy.” Mitophagy also removes mutated mtDNA and toxic ROS to prevent them from damaging other healthy mitochondria. Several human age-related disorders, such as Parkinson’s disease, Alzheimer’s disease, cardiomyopathies, and cancer, are resulting from dysregulation of mitophagy.
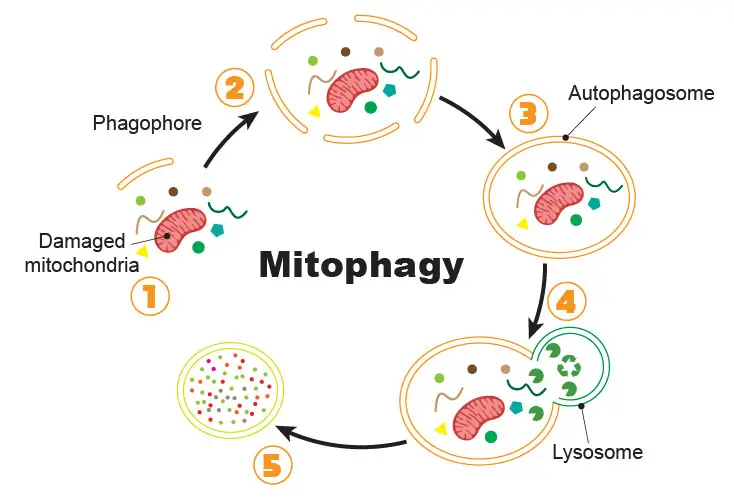
[In this figure] The process of mitophagy.
(1) Once the cell initiates the mitophagy, special lipid components (called phagophore) recruit close to the bad mitochondria tagged for recycling. (2) More and more phagophores gather around the cellular garbage. (3) Phagophores assemble into a complete vesicle, called the autophagosome. (4) Fusion with the lysosome brings in the digestive enzymes to break down the contents. (5) Contents are recycled as raw materials for the cells to use.
[In this video] Time-lapse STED imaging of the rupture of the mitochondrial membrane.
Source: Wang C., et al., PNAS.
Mitochondrial transfer and transplantation
Mitochondria are more dynamics than we think (maybe due to their endosymbiotic background). Sometimes, mitochondria can be shuttled between two cells. Mammalian cells can exchange their cellular contents, including molecules, RNA, and organelles (mitochondria and lysosomes) through a very thin tube connecting two cells. This tube is called tunneling nanotubes (TNT). In some other cases, the exchange of mitochondria achieves by sending small vesicles from one cell to another. The function and mechanism of these mitochondrial transfer are still unclear. It may involve how cells regenerate from an injury like a stroke.
[In this video] The mysterious nanotube network connecting cells.
Mitochondria can even be transplanted to treat severe heart diseases. In a 2018 NY times report, a medical group from Boston Children’s hospital isolated free mitochondria from a piece of muscle. They then injected mitochondria into the heart of an infant with a lethal congenital heart defect. Magically, this “mitochondrial transplantation” fixed the dying heart.

[In this image] Kate Bowen with her infant, Georgia, in the intensive care unit at Boston Children’s Hospital. Doctors tried to revive the baby’s heart with an infusion of one billion mitochondria.
Photo source: The New York Times.
Replacing bad mitochondria to cure mitochondrial genetic diseases
Mitochondrial disorders can be devastating. Leigh syndrome is a severe neurological disorder caused by specific mutations in mtDNA. While the mother was a carrier, she did not have neurological symptoms herself because only a fraction of her cells’ mitochondria carried damaged DNA. However, her baby may inherit a significant number of bad mitochondria and trigger Leigh syndrome.
Recently, a new technique based on in vitro fertilization (IVF) may be a solution. Basically, the damaged mitochondria in the mother’s egg were replaced by healthy mitochondria from another woman’s donor egg. The developing embryo now has nuclear DNA from the mother and father and mitochondrial DNA from the donor egg. This first child was referred to as a “three-parent baby” when he was born in Mexico in 2016.

[In this figure] Diagram of mitochondrial transfer by IVF.
Doctors first remove the genetic material from the donor egg (purple), leaving an empty egg full of healthy mitochondria and other nutrients. Next, doctors remove the genetic material from the mother’s egg (teal) and transfer it into the now empty donor egg. After the transfer, the newly engineered egg (purple + teal) is fertilized with the father’s sperm (dark teal) and then implanted into the mother’s womb.
The use of mitochondrial transfer is controversial as there are still several safety concerns. The scientific community will closely follow any child conceived using the mitochondrial transfer to determine if it is safe to continue using.
Q&A
How many mitochondria are in a cell?
The numbers of mitochondria can reflect the energy demand of the cell type. For example, heart muscles host more mitochondria to power the heart bumping. A heart muscle cell (or cardiomyocyte) can have about 40% of the cytoplasmic space taken up by ~ 5,000 mitochondria. Liver cells, which carry out many biochemical reactions, also have high mitochondrial content (about 20-25% space with 1000 to 2000 mitochondria per cell). Normal cells may contain 100-500 mitochondria. On the other hand, our red blood cells lose their mitochondria and nuclei so that they can carry more oxygen.

[In this figure] A transmission electron micrograph showing a human skeletal muscle cell.
Wide red bands represent the myofibrils, while mitochondria (green) supply the energy necessary for muscle contraction.
Photo source: Britannica.
The distribution of mitochondria inside a cell also reflects the local demand. For instance, cardiomyocytes’ mitochondria are packed along each myofibril (long filaments that generate contraction force) to ensure energy supply. The tail of a sperm contains 50-70 mitochondria, which form a spiral-like structure along the length of the tail. In nervous cells, mitochondria can be transported for a long distance to support the neurotransmission activities at the end of axons.
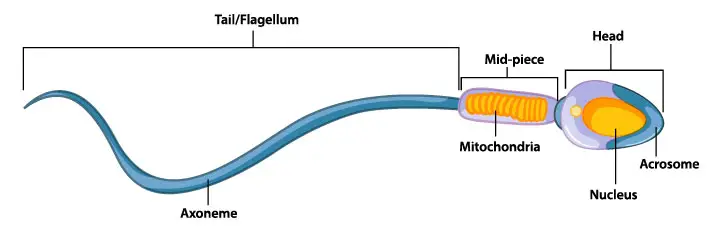
[In this figure] Diagram of human sperm showing the spiral-like mitochondria.
[In this video] Mitochondria trafficking in neurons.
What does mitochondrion look like?
The sausage shape in the textbook drawing has almost become the conventional impression for a mitochondrion. In fact, the shape of mitochondria can vary significantly in different cells or the same cells but different states. In epithelial or endothelial cells, mitochondria are long filaments, while in embryos, they tend to be more spherical. They can also form spirals, as seen in the tail of sperm. Mitochondria change their shape quite quickly. They also join up (fusion) and then split up (fission) again as needed.
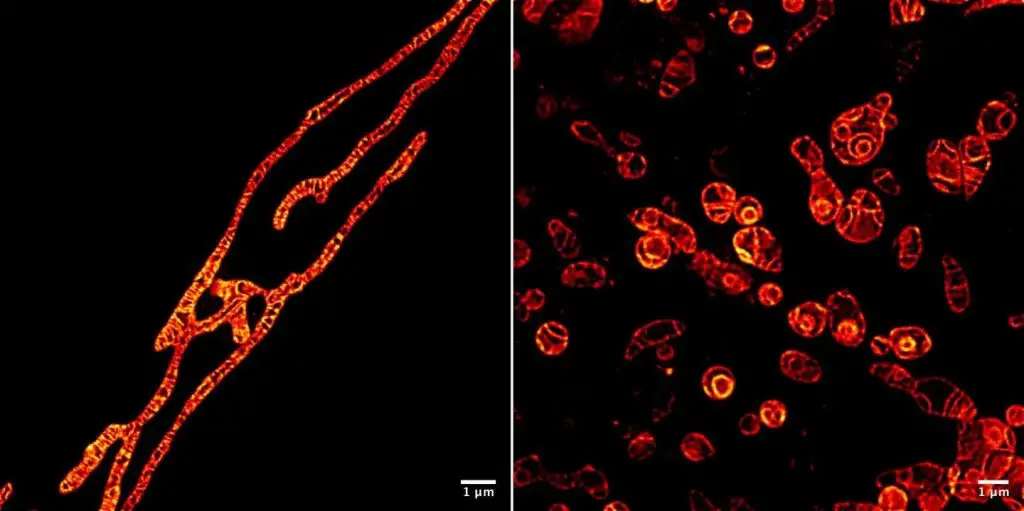
[In this figure] Stimulated emission depletion (STED) microscopy showing long or spherical mitochondrial morphology with a super-resolution.
Photo credit: Wang C., et al., PNAS.
[In this video] Rapid change of mitochondrial cristae morphology under a STED microscope.
Source: Wang C., et al., PNAS.
[In this video] Super-resolution (STED) imaging of mitochondria (green) and ER (red) in fibroblasts.
A mitochondrion is typically about 0.5-1 μm in diameter (about the sizes of bacteria). After staining, it looks like ‘threads dotted with grains’ under a good light microscope. The name “mitochondrion” is derived from the Greek word “mitos” meaning “thread,” and “-chondrion” meaning a “grain.”
Where did mitochondria come from – endosymbiotic theory
Scientists believe mitochondria and chloroplasts are derived from the bacteria engulfed by today’s eukaryotic cells’ early ancestors. This theory is called the endosymbiotic theory.
Around 1.5 billion years ago, some prokaryotic cells engulfed other prokaryotes into their cells. These engulfed cells were not digested and stayed in a symbiotic relationship. These incorporated prokaryotes then lost their ability to live independently and become integrated as part of the hosts. They later evolved to provide the host with specific functions, such as mitochondria and chloroplasts specialized in energy production. The host cell provides physical protection and a constant supply of food and oxygen in return.
Mitochondria appear to be related to Rickettsiales proteobacteria, and chloroplasts appear to be related to nitrogen-fixing filamentous cyanobacteria. Both mitochondria and chloroplasts still keep their own DNA to make some of their proteins, but most of their proteins still require nuclear DNA from the host cells.
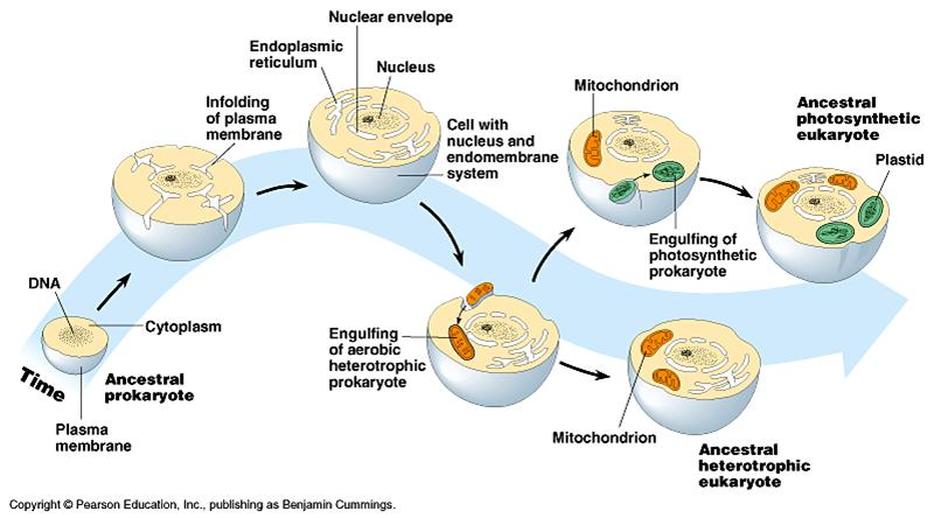
[In this figure] Overview of the process of endosymbiosis.
Source: Mysciencesquad classes.
The double layers of mitochondrial membranes are another evidence of endosymbiotic origin. The IMM could be the original membrane of the engulfed bacterium. The OMM was the remained vesicle when the host cell incorporated the bacterium. The engulfing process is similar to the “phagocytosis” of Amoeba.
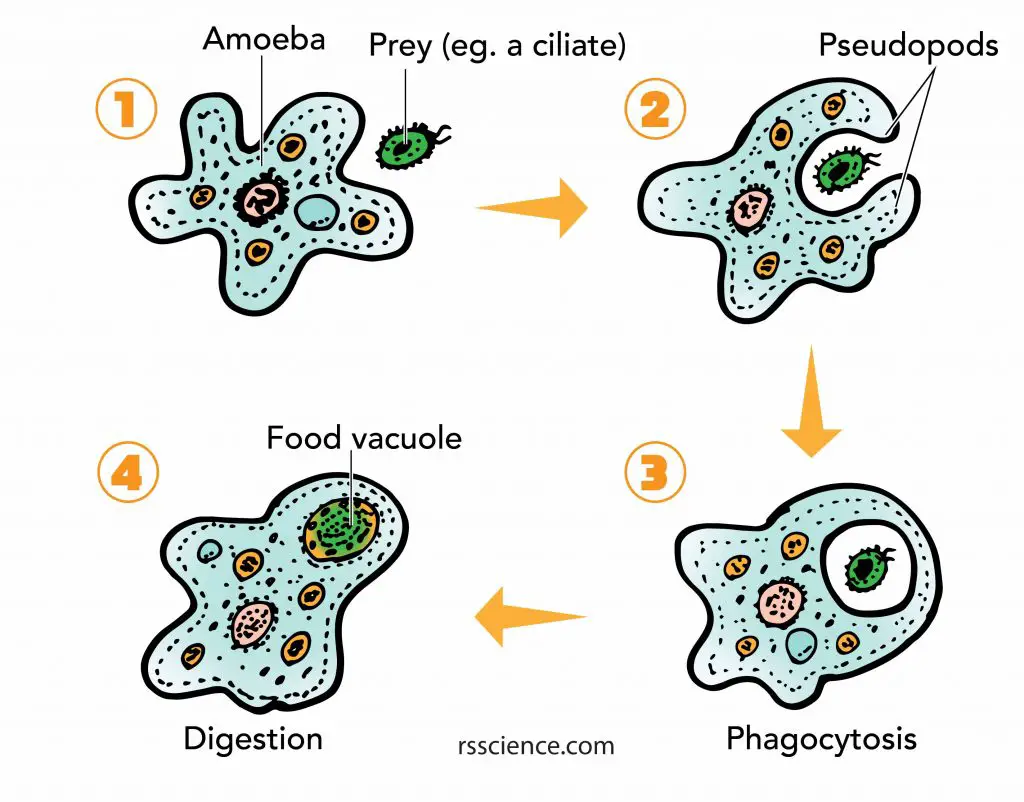
[In this figure] Phagocytosis of Amoeba cell.
Do plant cells have mitochondria?
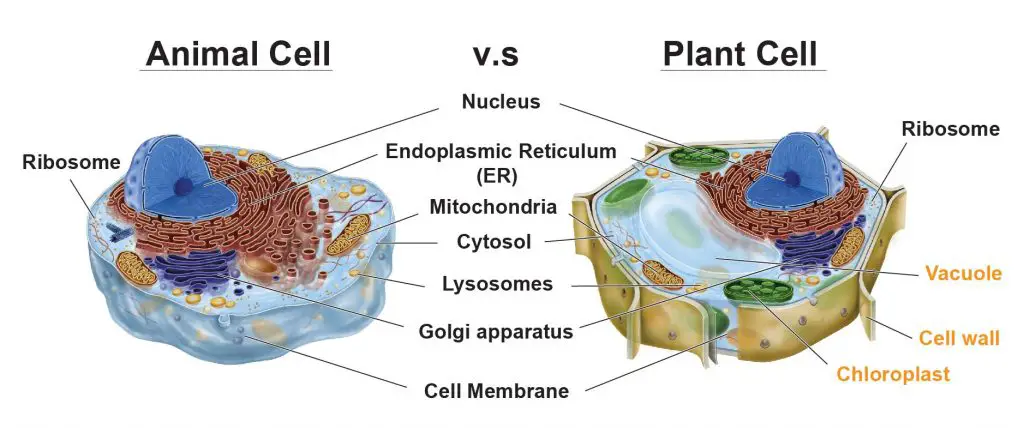
Both animal and plant cells have mitochondria, but only plant cells have chloroplasts. In the plant cells, chloroplasts absorb energy from sunlight and store it in sugars (photosynthesis). In contrast, mitochondria use chemical energy stored in sugars as fuels to generate ATP (cellular respiration). Like animal cells, plant cells use ATP to drive other cellular activities.
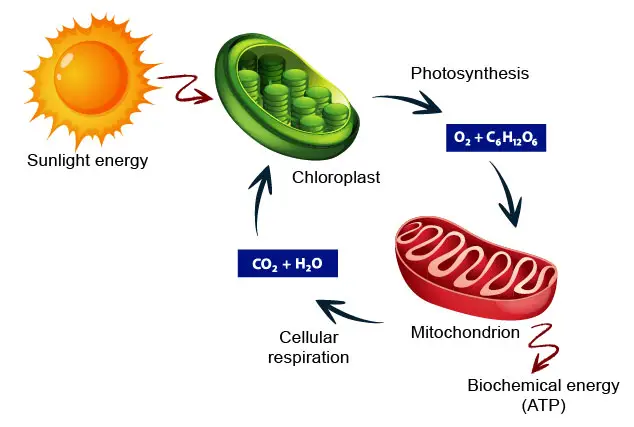
[In this figure] The carbon cycle showing how energy flows between chloroplasts and mitochondria to benefit the ecosystem.
In other words, plant cells have three copies of the DNA genome: nuclear DNA (nDNA), mitochondrial DNA (mtDNA), and chloroplast DNA (cpDNA). cpDNA is also known as the plastome when referring to genomes of other plastids.
Do prokaryotes have mitochondria?
Prokaryotes (bacteria and archaea), on the other hand, don’t have mitochondria for energy production, so they must rely on their immediate environment to obtain usable energy. Prokaryotes have their ATP synthesis machinery embedded in the cell membrane. This could be the original prototype of electron transport chains that we found today in eukaryotes’ mitochondrial inner membrane (another evidence of endosymbiotic theory).
Would an animal cell survive without mitochondria?
At the organism level, the answer is no. Without mitochondria, animals will not survive because the energy provided by anaerobic respiration is not enough to support the demand. We can learn this by looking at several genetic disorders that cause by mutations in mtDNA. Most of these diseases are severe or lethal because the powerhouse function of mitochondria is impaired.
However, if what you ask is at the cellular level, the answer may be “Yes”. In the laboratory, scientists can intentionally culture a cell line lack mtDNA. This can be achieved by a slow removal of mtDNA by chemical treatment. These mtDNA-depleted cells (called ρ0 or Rho-zero cells) can only survive with special nutrient supplements and yield scientific insight into physiological mechanisms of mitochondria biology.
Some cancer cells can be abundant in their aerobic respiration and rely largely on glycolysis and fermentation to obtain energy. This is called Warburg Effect. To do so, these cancer cells will plunder more glucose from the microenvironment to sustain themselves.
Summary
- Mitochondrion (plural: mitochondria) is a rod-shaped organelle and is considered the power generator of the cell.
- Mitochondrion performs cellular respiration, which converts glucose and oxygen to adenosine triphosphate (ATP). ATP is the biochemical energy “currency” of the cell for all activities.
- A mitochondrion has double layers of the membrane: outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM). Between the OMM and IMM is the intermembrane space. The region inside the inner membrane is called the matrix. These are many folds of the inner membrane, called cristae.
- Mitochondrion generates ATP like a hydraulic dam. It happens via the electron transport chain across the IMM.
- Mitochondria (in plant cells, chloroplasts, too) are the only organelles with their own DNA other than the nucleus. Mitochondrial DNA (mtDNA) is circular and encoded only 13 proteins.
- Scientists believe mitochondria and chloroplasts are derived from the bacteria engulfed by today’s eukaryotic cells’ early ancestors. This theory is called the endosymbiotic theory.
- Dysfunctional mitochondria cause many diseases. The cells use the fusion-fission cycle to sort out damaged mitochondria and eliminate them by mitophagy.
Fun Science
See how we build edible cell models with cherry tomato mitochondria.
References
“Mitochondrion – much more than an energy converter” by British Society for Cell Biology
“The Common and Distinct Features of Brown and Beige Adipocytes”
“Brown Adipose Tissue Development and Metabolism”
“Mitochondrial Regulation of Cell Death”
“Humanin: a harbinger of mitochondrial-derived peptides?”
“Principles of Clinical Molecular Biology”
“Different Ancestry Information By Gender”
“Dying Organs Restored to Life in Novel Experiments”
“Mitochondrial Transfer: The making of three-parent babies” by Catherine Weiner