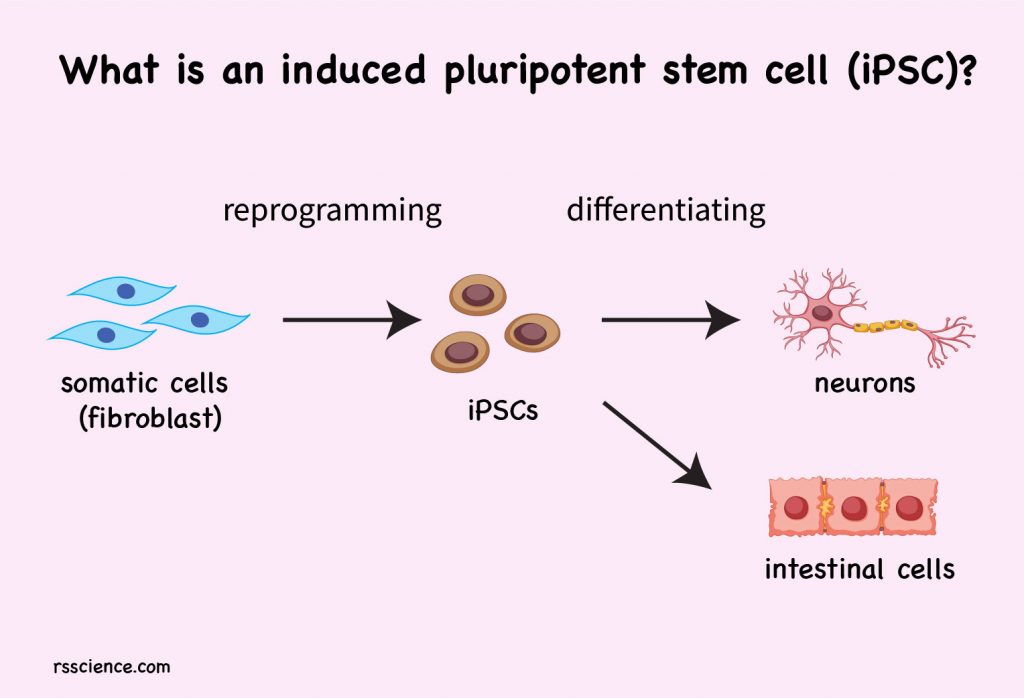
Induced pluripotent stem cells (iPSC) are among the most exciting discoveries in biomedical science in the past 20 years. You probably heard its name many times on the news. In this article, we will briefly explain to you what iPSC is and why it is so important in the future of medicine.
Stem cells: What they are and what they do?
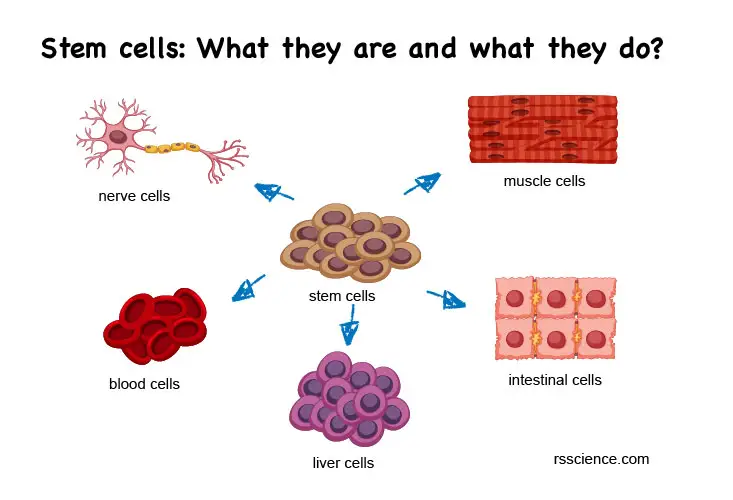
Before we dive into iPSCs, we quickly review what “Stem cell” means.
Cells are the basic units of our body. Each cell type has its specialized functions. For example, neurons (or nervous cells) sense and transmit signals. Cardiomyocytes (or heart muscle cells) contract regularly to pump blood throughout the body. There are about 200 different types of cells in the body. All different types of cells originally come from the pluripotent stem cells in the embryo. The name “pluripotent” means they can become anything.
You can think that stem cells are the body’s raw materials. They can become (the scientists like to use the word “differentiate”) any cell types that our body needs at a particular time, in a specific place. When the embryo grows its tiny heart, the stem cells in the chest chamber naturally generate more and more cardiomyocytes. In a growing body, you will see stem cells divide and differentiate into specialized cells everywhere. These stem cells are guided by their neighbors and local environments to tell them what types of cells they should become.
At the same time, our body also reserves a proportion of untouched stem cells. These stem cells divide but do not differentiate (we called self-renewal). They keep the capability to become other cell types in the future to prepare for more growth or as a backup to repair damaged organs.
However, when we are getting older, we lose our reserve of stem cells. The ability to repair wounds declined, which is a major cause of many aging and metabolic-related diseases, including heart attack, diabetes, and neurodegenerative diseases. If we can replenish the old and damaged cells in our body, we may fundamentally treat many diseases. This concept is called “Regenerative Medicine,” obtaining these powerful stem cells for replenishment will be the key to success.
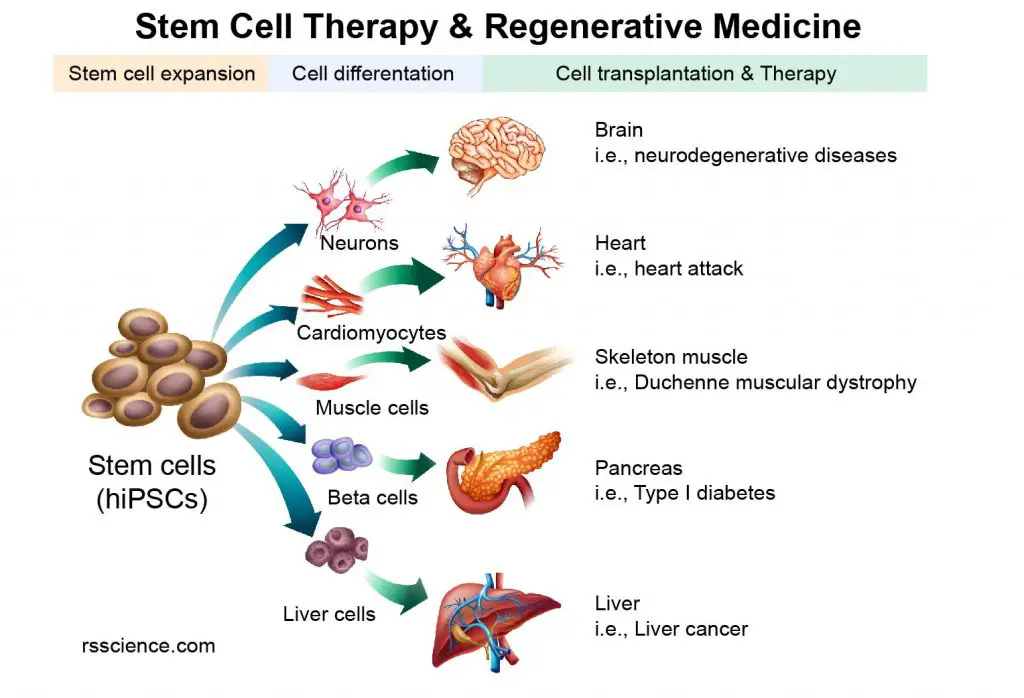
[In this image] The concept of Stem Cell Therapy and Regenerative Medicine.
Since our body is built by many single cells, diseases occur when cells are old, injured, and dead. If we can replenish these lost cells, we may be able to treat the diseases. This is the central concept of Regenerative Medicine. With stem cells in our hands, we can guide them to become the cell types we need for cell transplantation.
Induced pluripotent stem cells (iPSCs) make stem cell therapy possible
We cannot randomly get the cells from person A and immediately give them to patient B. The immune system in our bodies will destroy any cells that do not belong to us, called immune rejection. So, where can we get our own stem cells?
We do have some adult stem cells reserved in the bone marrow and fat. However, compared with embryonic stem cells, adult stem cells have a limited ability to give rise to various cells types in the body. Adult stem cells may not be able to provide the crucial cell types we need (like neurons, pancreatic beta cells, and cardiomyocytes).
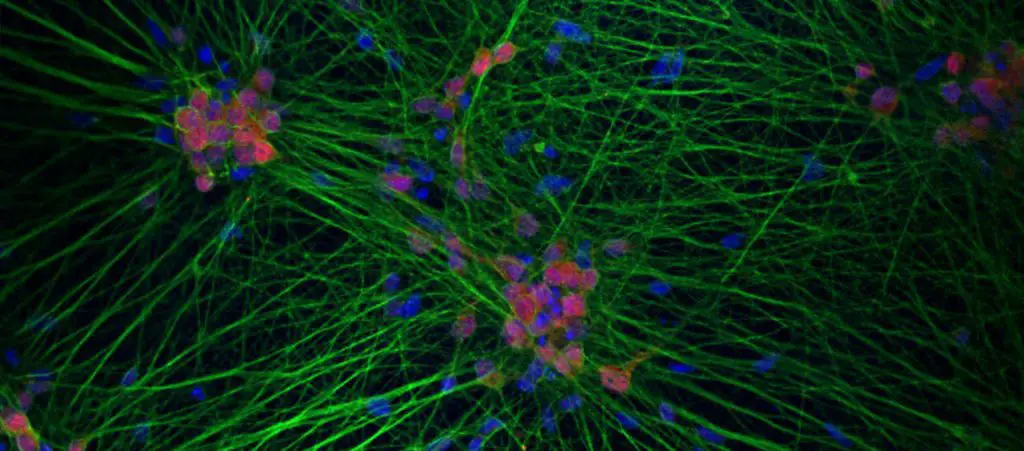
[In this image] Neurons are differentiated from human iPSCs.
The nuclei are stained in blue (DAPI) and processes stains in green. The processes long and thin structures, we don’t call them axons or dendrites in this case, because it is not clear which process sends or receives signals.
Photo credit: V. Lagomarsino, Young-Pearse laboratory
[In this video] Cardiomyocytes or heart muscle cells generated from human iPSCs beating in a cell culture dish.
In 2006, Professor Shinya Yamanaka of Kyoto University, Japan, discovered a new way that allows us to manufacture our own pluripotent stem cells. This technique is called “induced pluripotent stem cells or iPSCs”. He first compared the differences between a stem cell and a somatic cell. He selected 24 genes as candidates that may define the “stemness” properties of stem cells. Then, he tried to put these genes one-by-one back to mature body cells to see which combination can turn mature cells into embryonic cells. This is how he found these four genes – Oct3/4, Sox2, c-Myc, and Klf4 (we now called them “Yamanaka factors”) that can reverse the cell fate.
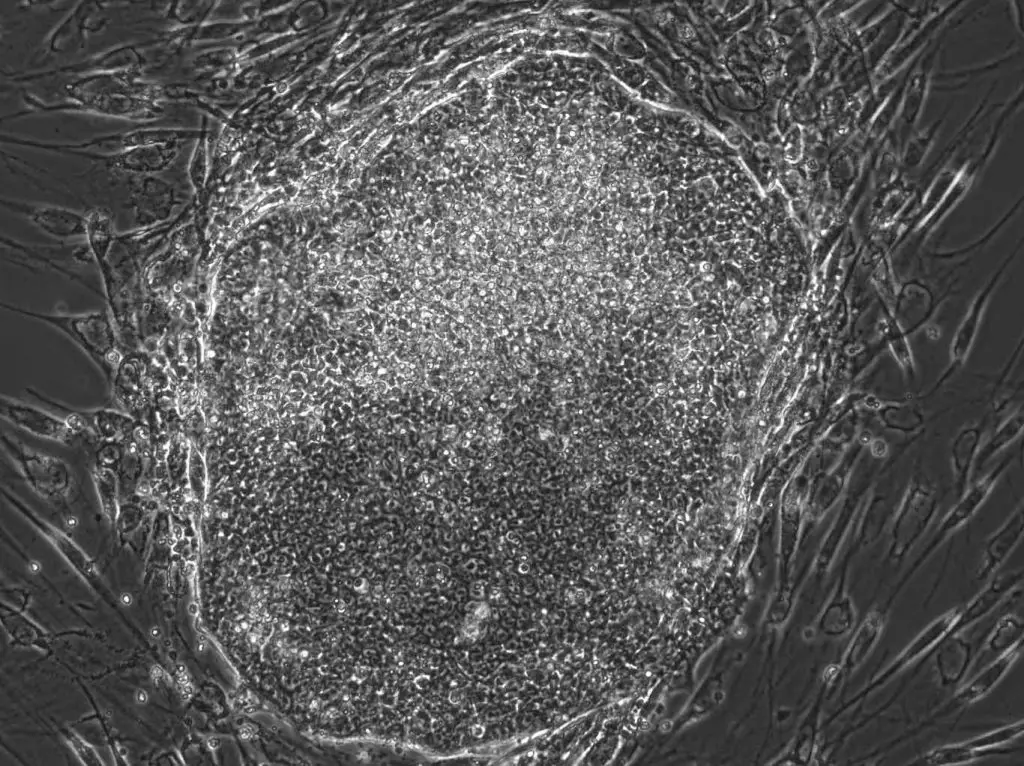
[In this image] A human iPSC colony I made in a laboratory. The original cells are human fibroblast cells from the skin. They are reprogramed to iPSCs by “Yamanaka factors” in 3 weeks.
These four genes work together can teach adult mouse cells to “forget” their differentiation state and be reprogrammed to an embryonic-like state. These embryonic-like cells are what we called “Induced pluripotent stem cells or iPSCs”. In 2007, this principle was applied to the human adult cells, leading to the term “human induced pluripotent stem cells” (hiPSC). These cells, once given the right environment, can grow to any type of cells we need. Now, we may have unlimited cell sources to replenish the old and damaged cells in our bodies.

[In this image] Professor Shinya Yamanaka received The Nobel Prize in Physiology or Medicine 2012 for the discovery that mature cells can be reprogrammed to become pluripotent.
Photo credit: nobelprize.org
More research to do, please support our scientists
In the last decade, scientists have made tremendous progress in stem cell biology and regenerative medicine. They have found the receipt to grow hiPSCs to almost any cell type we need in our bodies, including blood cells, liver cells, muscle cells, neurons, bone cells, and even eggs and sperms. Recently, they are learning how to grow a whole tissue or organ in a dish (called organoids). At the same time, we also discover how to correct mutated genes by CRISPR gene editing. The combination of iPSCs and gene editing may fix many genetic diseases by providing healthy cells for patients. All these developments are still in a very early stage, but their therapeutic potentials are enormous. In fact, there are several stem cell therapies already in the clinical trials. The actual clinical usages of stem cells may come in 5-10 years.
[In this video] Driving Towards a Cure for Diabetes: Stem Cell Science at the Wheel.
Researchers from Harvard Medical School are trying to use stem cells to cure diabetes and other diseases.
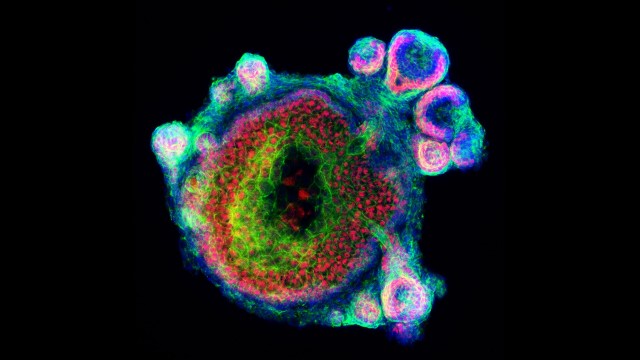
[In this image] A lab-grown skin organoid with hair follicles.
Photo credit: Karl Koehler lab from the Indiana University School of Medicine
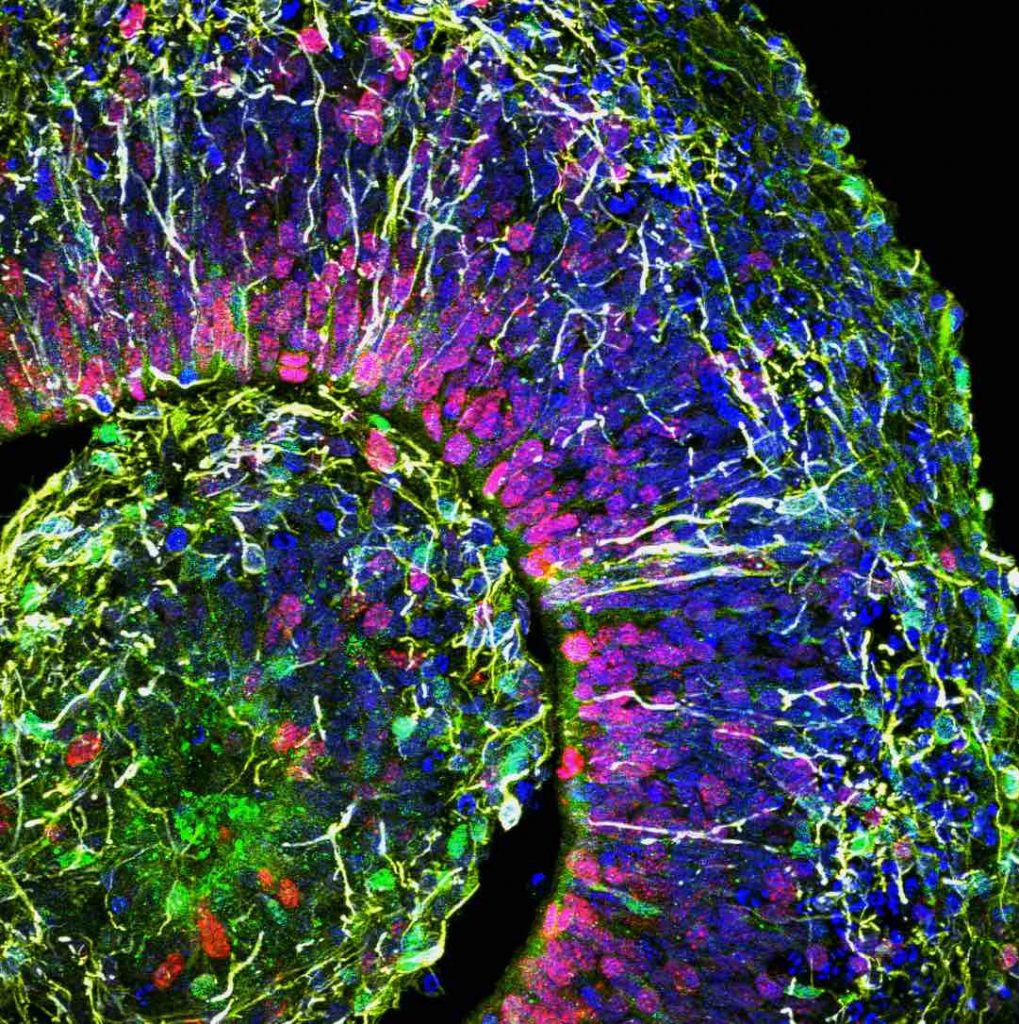
[In this image] A human brain organoid grown from stem cells.
Photo credit: Alysson Muotri lab from the University of California, San Diego