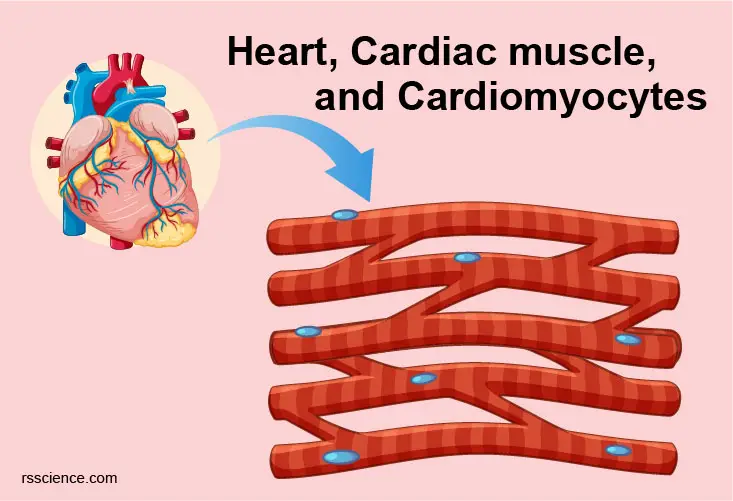
This article covers
Definition: What are cardiomyocytes?
Cardiac muscle cells or cardiomyocytes (also known as cardiac myocytes) are the muscle cells (myocytes) that make up the heart muscle. Cardiomyocytes go through a contraction-relaxation cycle that enables cardiac muscles to pump blood throughout the body.

[In this image] Immunostaining of human cardiomyocytes with antibodies for actin (red), myomesin (green), and nuclei (blue).
Photo source: https://www.fujifilmcdi.com/products/cardiac-cells/icell-cardiomyocytes
Cardiomyocytes are highly specialized cell types in terms of their structures and functions. Each cardiomyocyte contains myofibrils, unique organelles consisting of long chains of sarcomeres, the fundamental contractile units of muscle cells.
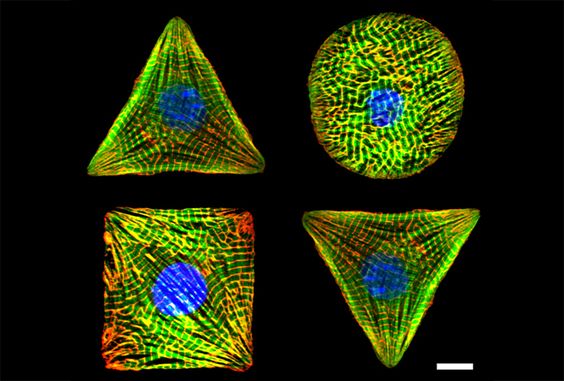
[In this image] Cardiomyocyte geometry and cellular architecture are controlled by micropatterned ECM substrate. Scientists used this technique to study how cells sense and respond to mechanical forces.
Photo source: https://diseasebiophysics.seas.harvard.edu/research/mechanotransduction/
Quick overview: Heart function and anatomy
The heart is a muscular organ that pumps blood through the blood vessels of the circulatory system. It is composed of individual heart muscle cells (cardiomyocytes) and several other cell types.
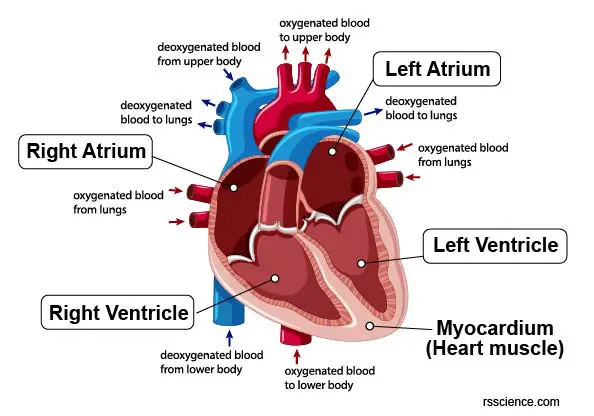
[In this figure] The anatomy of the human heart showing 4 heart chambers (left atrium, left ventricle, right atrium, right ventricle) and the blood flow. The myocardium is referred to the cardiac muscle layers building the wall of each chamber.

[In this figure] The thickness of the heart wall (or myocardium) consists of cardiac muscle cells.
Photo source: biologydictionary
[In this video] Structure of the human heart.
Why cardiomyocytes are important?
Cardiovascular disease is a leading cause of death worldwide. Nearly 2,400 Americans die of cardiac causes each day, one death every 37 seconds.
As the chief cell type of the heart, cardiac muscle cells primarily dedicate to the contractile function of the heart and enable the pumping of blood around the body. If anything goes wrong in the heart, it can lead to a catastrophic outcome. A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow ceases to a part of the heart, causing massive cardiomyocyte death in that area. Severe cases can, ultimately, lead to heart failure and death.
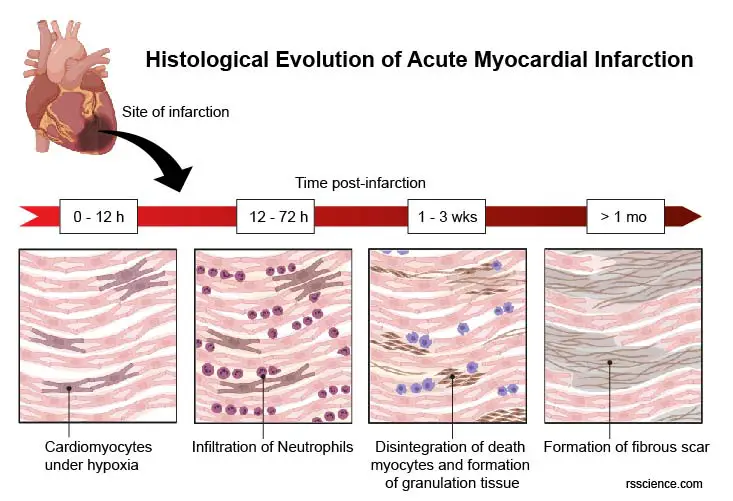
[In this figure] The progress of myocardial infarction or heart attack. At time post-infarction:
0-12 hours: Beginning of necrotic coagulation due to the blockage of coronary arteries – Cardiomyocytes suffer the lack of oxygen (hypoxia)
12-72 hours: Culmination of necrotic coagulation – Neutrophils infiltrate by an inflammatory response.
1-3 weeks: Disintegration of death myocytes and formation of granulation tissue (collagenous fibers, macrophages, and fibroblasts)
> 1 month: Formation of fibrous scar (fewer cells with an abundance of collagenous fibers)
How many cardiomyocytes are in the human heart? Are they alone?
A human heart contains an estimated 2–3 billion cardiomyocytes. There are several non-myocyte populations in the heart, including endothelial cells, smooth muscle cells, myofibroblasts, epicardial cells, endocardial cells, valve interstitial cells, resident macrophages, and other immune system-related cells, and potentially, adult “stem cells” (mesenchymal stem cells and cardiac stem cells). These distinct cell pools are not isolated from one another within the heart but interact physically to maintain the function of the whole organ. Overall, cardiomyocytes only account for less than a third of the total cell number in the heart.
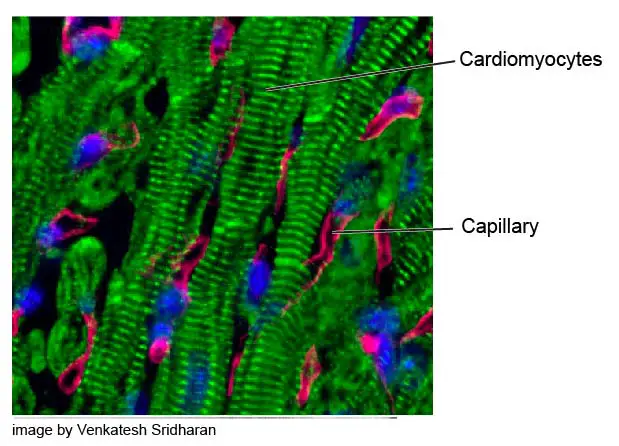
[In this image] Immunostaining showing highly vascularized heart muscle.
Cardiomyocytes are labeled by the striated pattern of sarcomeric α-actinin (green). Capillaries are red and nuclei are blue.
Photo source: biocompare.
What are the three different types of muscles in the human body?
The three main types of muscle include: Cardiac muscle, Skeletal muscle, and Smooth muscle.
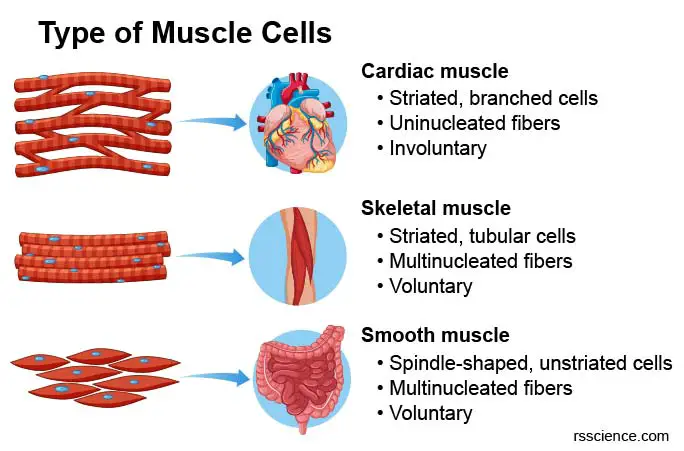
[In this figure] Morphology and comparison of cardiac, skeleton, and smooth muscles.
List of the difference between three muscle cell types
| Cardiac muscle | Skeletal muscle | Smooth muscle |
| Striated, branched cells | Striated, tubular cells | Spindle-shaped, unstriated cells |
| Uninucleated fibers | Multinucleated fibers | Uninucleated fibers |
| Involuntary | Voluntary | Involuntary |
| Forming the muscle walls of the heart and pumping blood to circulatory system. | Specialized tissue that is attached to bones and allows movement. | Located in various internal organs including the digestive tract, uterus and blood vessels to build the muscle walls. |
Note: Involuntary muscles are the muscles that cannot be controlled by will or conscious.
Two types of cardiac cells in the heart
There are two types of cells within the heart: the cardiomyocytes and the cardiac pacemaker cells.
- Cardiomyocytes are the true cardiac muscle cells that build up the muscle walls (called myocardium) of both atria (the chambers in which blood enters the heart) and the ventricles (the chambers where blood is collected and pumped out of the heart). These cells can shorten and lengthen their myofibers to create the pumping force of the heart. Human cardiomyocytes are about 100 μm long and 10-25 μm in diameter.
- Cardiac pacemaker cells are modified cardiomyocytes and control the beating of the heart. They generate and send out electrical impulses to cardiomyocytes spontaneously. Pacemaker cells create the rhythmic impulses of cardiac contraction. In other words, they directly control the heart rate.
What are the special features of cardiomyocytes?
The heart is composed of cardiac muscle cells that have specialized features that relate to their function:
- Like skeletal muscle, cardiac muscle is striated with narrow dark and light bands, due to the parallel arrangement of actin and myosin filaments.
- Cardiomyocytes do not fuse together. Thus, a majority of cardiomyocytes have a single nucleus (skeletal muscle cells have multiple nuclei).
- Cardiomyocytes are narrower and much shorter in comparison with skeletal muscle cells.
- Cardiac muscle cells are branched, allowing for faster signal propagation and contraction in three dimensions
- Cardiomyocytes closely connect with each other through intercalated discs.
- Cardiac muscle consists of interlacing bundles of cardiomyocytes.
- Cardiomyocytes have a high density of mitochondria than other cells to meet their metabolic demands.
These structural features contribute to the unique functional properties of the cardiac tissue:
- Cardiac muscle has a longer period of contraction and refraction, which is needed to maintain a viable heartbeat.
- The heart tissue does not become fatigued (unlike skeletal muscle), allowing for continuous, life-long contractions.
- The interconnected network of cells is separated between atria and ventricles, allowing them to contract separately.
Ultrastructure and organelles of cardiomyocytes
Like other animal cells, cardiomyocytes contain all the cell organelles that are essential for normal cell physiology. Moreover, cardiomyocytes have several unique cellular structures that allow them to perform their function effectively. Here are five main characteristics of mature cardiomyocytes: (1) striated; (2) uninucleated; (3) branched; (4) connected by intercalated discs; (5) high mitochondrial content.
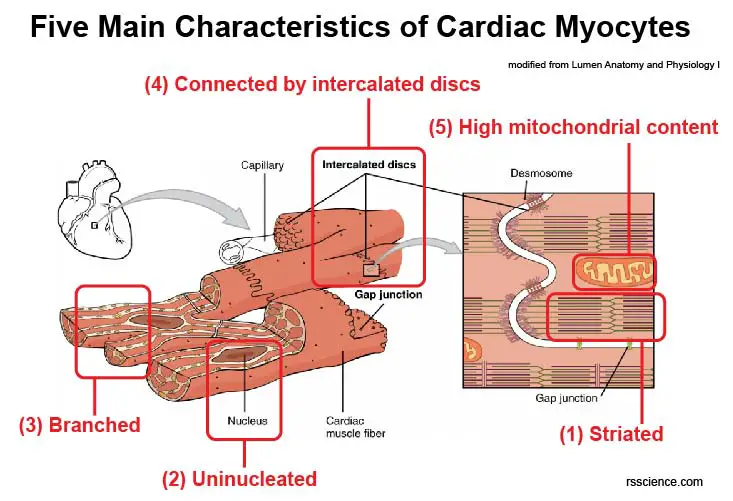
[In this figure] Main characteristics of cardiac myocytes.
Modified from lumen Anatomy and Physiology I.
Let’s get closer to look inside a cardiomyocyte and learn its unique ultrastructure.
A. Intercalated discs
All cardiomyocytes and pacemaker cells are linked by cellular bridges. Intercalated discs, which form porous junctions, bring the membranes of adjacent cardiomyocytes very close together. These pores (gap junctions) permit ions, such as sodium, potassium, and calcium, to easily diffuse from cell to cell, establishing a cell-cell communication. This joining is called electric coupling, and it allows the quick transmission of action potentials and the coordinated contraction of the entire heart.
Intercalated discs also function as mechanical anchor points that enable the transmission of contractile force from one cardiomyocyte to another (by desmosomes and adherens junctions). This allows for the heart to work as a single coordinated unit.

[In this figure] Cardiac muscle cells are connected together to coordinate the cardiac contraction. This joining is called electric coupling and is achieved by the presence of irregularly-spaced dark bands between cardiomyocytes. These bands are known as intercalated discs.
Photo source: bioninja.
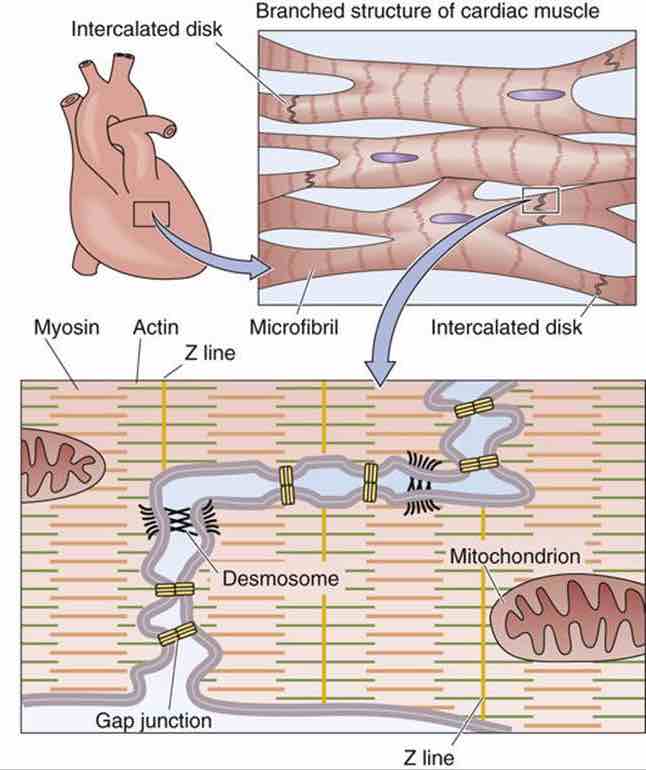
[In this figure] Cardiac myocytes are branched and interconnected from end to end by structures called intercalated disks, visible as dark lines in the light microscope.
Photo source: https://doctorlib.info/physiology/medical/49.html
There are 3 main types of junctional complexes within the intercalated discs. They work in different ways to maintain cardiac tissue integrity and cardiomyocyte synchrony.
(1) Desmosomes
The term “desmosome” came from Greek words of bonding (desmo) and body (soma). Desmosomes serve as the anchor points to bring the cardiac muscle fibers together. Desmosomes can withstand mechanical stress, which allows them to hold cells together. Without desmosomes, the cells of the cardiac muscles will fall apart during contraction.
The ability of desmosome to resist mechanical stress comes from its unique 3-D structure. Desmosome is an asymmetrical protein complex bridging between two adjacent cardiomyocytes, with each end residing in the cytoplasm. The intracellular part anchors intermediate filaments in the cytoskeleton to the cell surface. The middle part bridges the intercellular space between two cytoplasmic membranes.
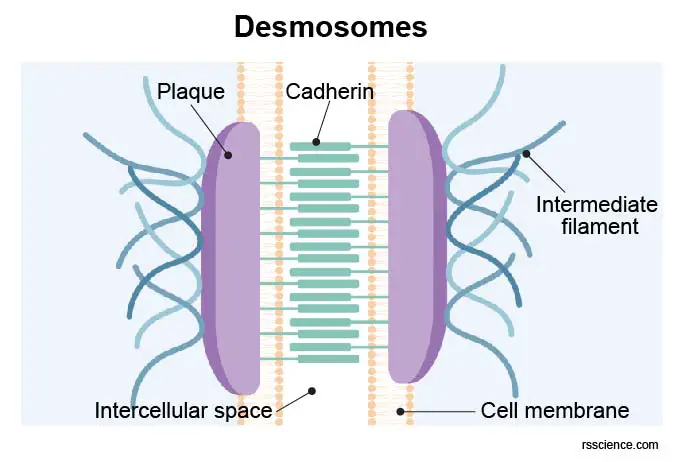
[In this figure] Desmosomes connect intermediate filaments from two adjust cardiomyocytes. This job is accomplished by the formation of a dense protein complex or plaque in the intercalated discs. Major protein players include transmembrane cadherins: desmogleins (Dsgs) and desmocollins (Dscs), cytoplasmic anchors: plakophilins (PKPs) and plakoglobin (PG), and cytoskeleton adaptor: desmoplakin (DP). Cadherins link cells together, and other proteins form a dense complex called plaque.
(2) Adherens junctions (also called fascia adherens in cardiac muscle)
In addition to desmosomes, adherens junctions (Ajs) are another type of mechanical intercellular junctions in cardiomyocytes. The difference is that adherens junctions link the intercalated disc to the actin cytoskeleton and desmosomes attach to intermediate filaments.
Adherens junctions keep the cardiac muscle cells tightly together as the heart pump. Adherens junctions are also the anchor point where myofibrils are attached, enabling transmission of contractile force from one cell to another.
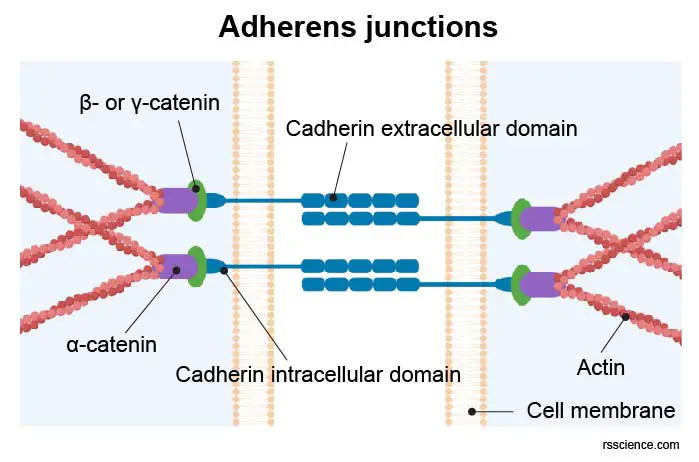
[In this figure] Adherens junctions link actin cytoskeleton from two adjacent cardiomyocytes together.
Adherens junctions are constructed from cadherins and catenins. Cadherins (in cardiomyocytes N-Cadherin is the main cadherin) are transmembrane proteins that zip together adjacent cells in a homophilic manner. The transmembrane cadherins form complexes with cytosolic catenins, thereby establishing the connection to the actin cytoskeleton. At the adherens junctions, the opposing membranes become separated by ∼ 20 nm.
(3) Gap junctions
Gap junctions are essential for the chemical and electrical coupling of neighboring cells. Gap junctions work like intercellular channels connecting the cytoplasm of neighboring cells, enabling passive diffusion of various compounds, like metabolites, water, and ions, up to a molecular mass of 1000 Da. Thereby they establish direct communication between adjacent cells.
[In this figure] Neonatal rat cardiac myocytes in cell culture.
Cells were immunostained for actinin (green), gap junctions (red), and counterstained with DAPI (blue).
Photo source: bioscience
Gap junctions are present in nearly all tissues and cells throughout the entire body. In cardiac muscle, gap junctions ensure proper propagation of the electrical impulse (from pacemaker cells to neighboring cardiomyocytes). This electrical wave triggers sequential and coordinated contraction of the cardiomyocytes as a whole.
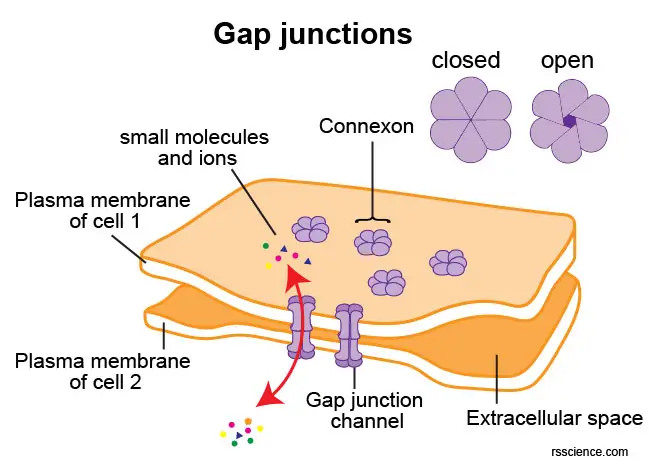
[In this figure] A gap junction channel consists of twelve connexin proteins, six of which are contributed by each cell. The six connexin subunits form a hemi-channel in the plasma membrane, which is called a connexon. A connexon docks to another connexon in the intercellular space to create a complete gap junction channel. The intercellular space between adjacent cells at the site of a gap junction is 2-4 nm.
B. Sarcomeres and myofibrils
A second feature of cardiomyocytes is the sarcomeres, which are also present in skeletal muscles. The sarcomeres give cardiac muscle their striated appearance and are the repeating sections that make up myofibrils.
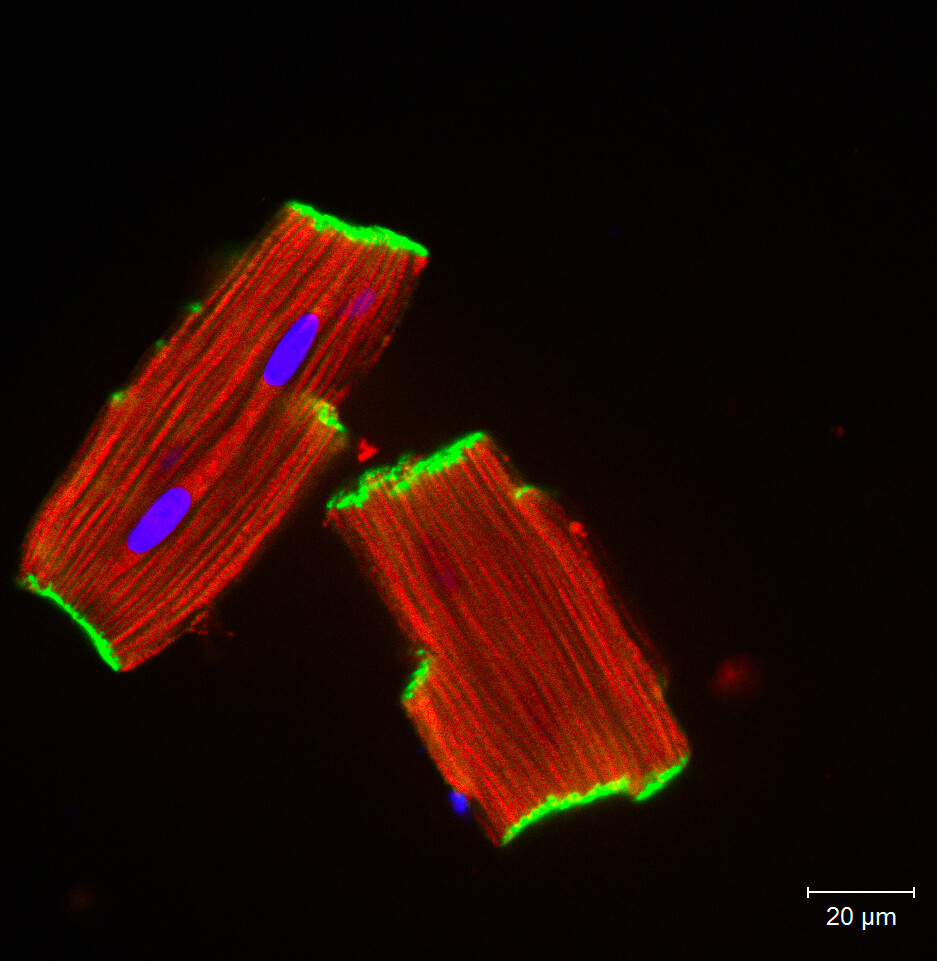
[In this image] Freshly isolated heart muscle cells showing intercalated discs (green), sarcomeres (red), and nuclei (blue).
Photo source: https://christianz.artstation.com/
Cardiac muscle cells are equipped with bundles of myofibrils that contain myofilaments. These fiber-like structures can occupy 45-60% of the volume of cardiomyocytes. The myofibrils are formed of distinct, repeating units, termed sarcomeres. The sarcomeres, which are composed of thick and thin myofilaments, represent the basic contractile units of a muscle cell and are defined as the region of myofilament structures between two Z-lines (see image below). The distance between Z-lines in human hearts ranges from around 1.6 to 2.2 μm.
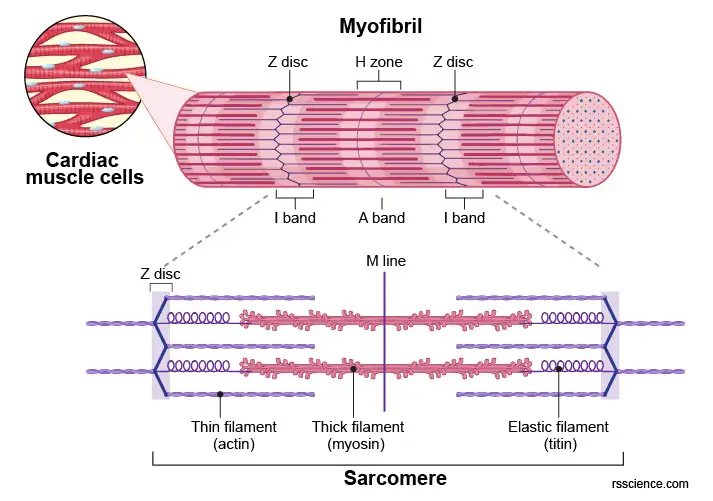
[In this figure] Labeled diagram of myofibril showing the unit of a sarcomere. A sarcomere is defined as a segment between two neighboring Z-discs.
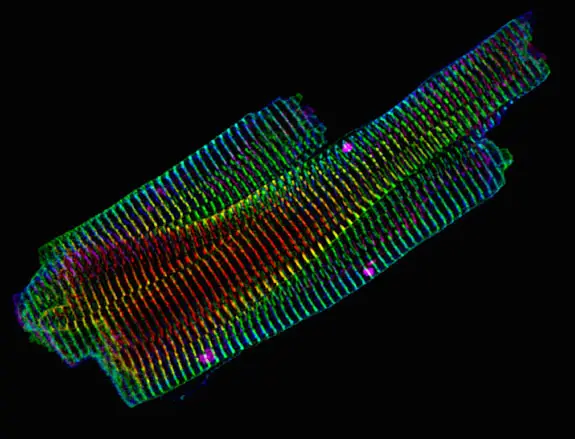
[In this image] Immunofluorescence image of adult mouse cardiomyocytes showing the “Z-lines” of the sarcomeres. 3D color projection of alpha-actinin 2 acquired with a confocal microscope.
Photo source: Dylan Burnette.
Sarcomeres contains two types of myofilaments:
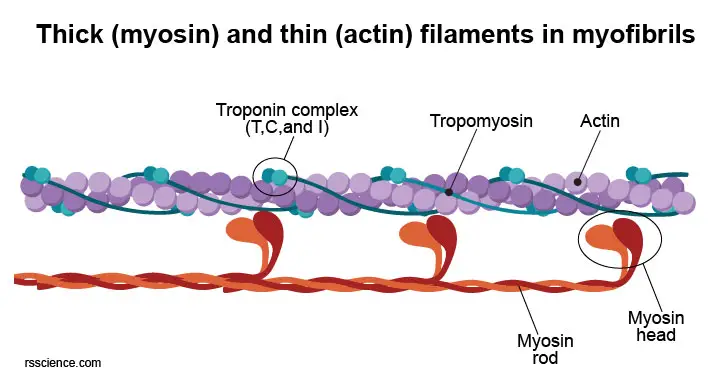
(1) The thick filaments (15 nm in diameter)
The thick filaments are composed of myosin II. Each myosin contains two ATPase sites on its head. ATPase hydrolyzes ATP and this process is required for actin and myosin cross-bridge formation. These heads bind to actin on the thin filaments. There are about 300 molecules of myosin per thick filament.
(2) The thin filaments (7nm in diameter)
The thin filaments are composed of single units of actin known as globular actin (G-actin). Two strands of actin filaments form a helix, which is stabilized by rod-shaped proteins termed tropomyosin. Troponin proteins, which function as regulators, bind to the tropomyosin at regular intervals. Whereas troponin lies in the grooves between the actin filaments, tropomyosin covers the sites on which actin binds to myosin. Their respective actions, therefore, control the binding of myosin to actin and consequently in the contraction and relaxation of cardiac muscles.
To generate muscular contraction, the myosin heads bind to actin filaments, allowing myosin to function as a motor that drives filament sliding. The actin filaments slide past the myosin filaments toward the middle of the sarcomere. This results in the shortening of the sarcomere without any change in filament length.
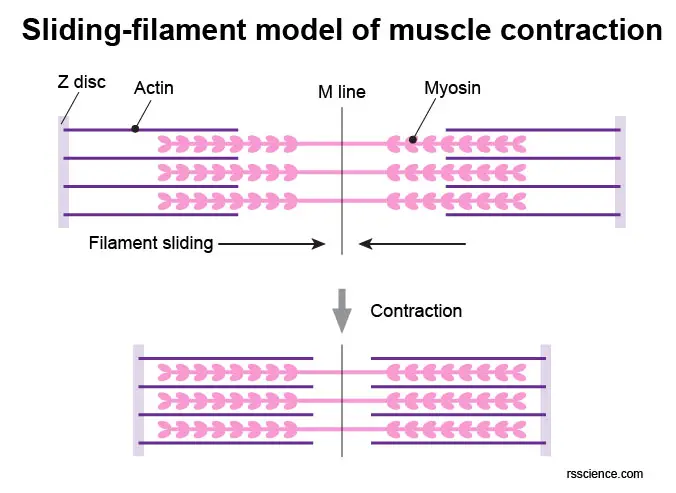
[In this figure] Sliding-filament model of muscle contraction.
C. Sarcolemma
Sarcolemma (also called myolemma) is a specialized cell membrane of cardiomyocytes and skeletal muscle cells. It consists of a lipid bilayer and a thin outer coat of polysaccharide material (glycocalyx) that contacts the basement membrane. The sarcolemma is also part of the intercalated disks as well as the T-tubules of the cardiac muscle.
D. Basement membrane
Basement membrane is an extracellular matrix (ECM) coat that cover individual cardiomyocytes. It’s composed of glycoproteins laminin and fibronectin, type IV collagen as well as proteoglycans that contribute to its overall width of about 50nm. Basement membrane provides a scaffold to which the muscle fiber can adhere.
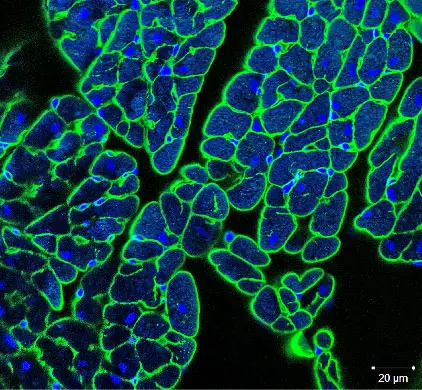
[In this figure] A cross-section of a mouse heart showing the basement membrane (green) wrapping around an individual myocyte.
E. T-tubules
In cardiomyocytes and skeletal muscle cells, the sarcolemma (i.e. the plasma membrane) forms deep invaginations known as T-tubules (or transverse tubules). These invaginations increase the total surface area and allow depolarization of the membrane to penetrate quickly to the interior of the cell.
Without t-tubules, the wave of calcium ions (Ca2+) takes time to propagate from the periphery of the cell into the center. This time lag will first activate the peripheral sarcomeres and then the deeper sarcomeres, resulting in sub-maximal force production.
The t-tubules make it possible that current is simultaneously relayed to the core of the cell, and trigger near to all sarcomeres simultaneously, resulting in a maximal force output. T-tubules also stay close to sarcoplasmic reticulum (SR) networks, which is the modified endoplasmic reticulum (ER) of calcium storage in myocytes.
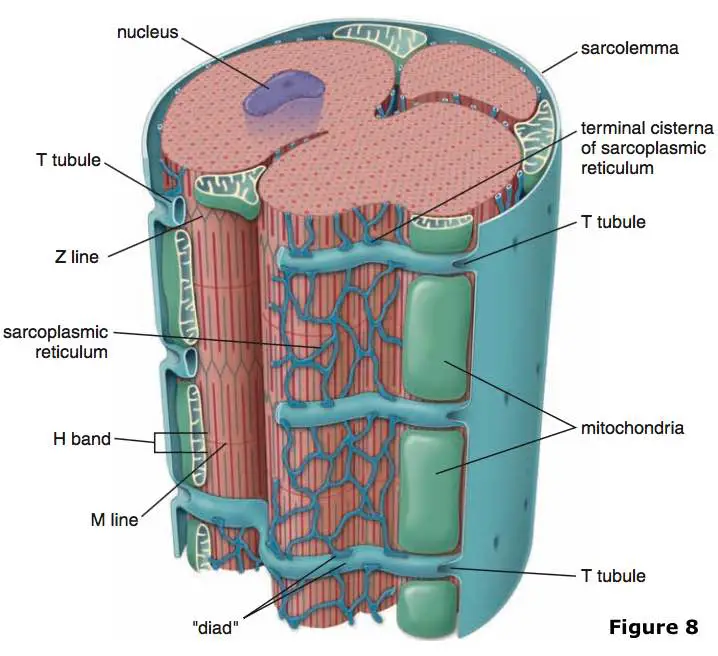
[In this figure] T-tubules (transverse tubules) are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. T-tubules permit the rapid transmission of the action potential into the cell and also play an important role in regulating cellular calcium concentration.
F. Mitochondrial morphology and energy metabolism in cardiomyocytes
Mitochondria are the “powerhouse of the cell” because they generate most of the cell’s energy supply of adenosine triphosphate (ATP). It is no doubt that the normal functions of cardiomyocytes require a lot of energy. Effective heart pumping is primarily dependent on oxidative energy production by mitochondria. Cardiomyocytes have a densely packed mitochondrial network, which allows them to produce ATP quickly, making them highly resistant to fatigue.
Different types of mitochondria can be distinguished within cardiomyocytes, and their morphological features are usually defined according to their location: intermyofibrillar mitochondria, subsarcolemmal mitochondria, and perinuclear mitochondria.
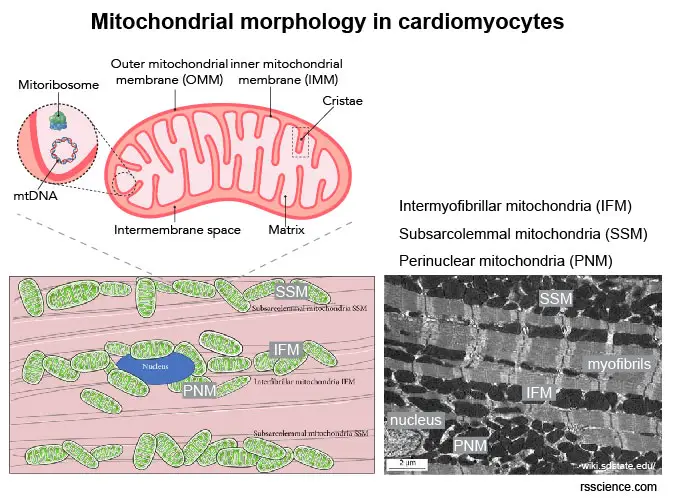
[In this figure] Mitochondrial morphology in cardiomyocytes.
(Top) The anatomy of a mitochondrion. (Bottom left) Schematic diagram of the location of subsarcolemmal mitochondria (SSM), interfibrillar mitochondria (IFM), and perinuclear mitochondria (PNM). (Bottom right) TEM images of mitochondria in cardiomyocytes.
Photo source: researchgate, wiki
Intermyofibrilar Mitochondria are found deeper within the cells and strictly ordered between rows of contractile proteins, apparently isolated from each other by repeated arrays. They play a huge role in producing enough energy for muscle contractions.
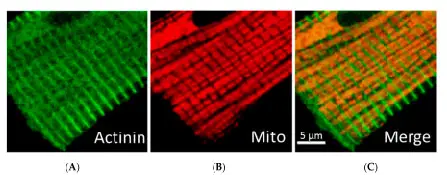
[In this figure] Immunofluorescent confocal imaging showing the densely packed mitochondria in cardiomyocytes. (A): Z-line (actinin); (B): Mitochondria; (C): Merge image.
Photo source: MDPI
Subsarcolemmal Mitochondria reside beneath the sarcolemma. They collect oxygen from the circulating blood in the arteries and are responsible for providing the energy needed for conserving the integrity of the sarcolemma.
Perinuclear mitochondria are organized in clusters around the nucleus to provide energy for transcription and translation processes.
High metabolic demands of cardiomyocytes
The cardiac function requires high energy demands; therefore, the adult cardiomyocytes contain numerous mitochondria, which can occupy at least 30% of cell volume. They meet >90% of the energy requirements by oxidative phosphorylation (OXPHOS) in the mitochondria, which requires a huge demand for oxygen consumption.
In humans, at a heart rate of 60–70 beats per minute, the oxygen consumption of the myocardium is 20-fold higher than that of skeletal muscle at rest (compared by a normalization per gram of cell mass). In order to meet this high oxygen demand, the capillary density in the heart is 2-8 times higher than that in skeletal muscle (3,000–4,000/mm2 compared to 500–2,000 capillaries/mm2, respectively). Also, cardiomyocytes maintain a very high level of oxygen extraction (from blood) of 70–80% compared with 30–40% in skeletal muscle.
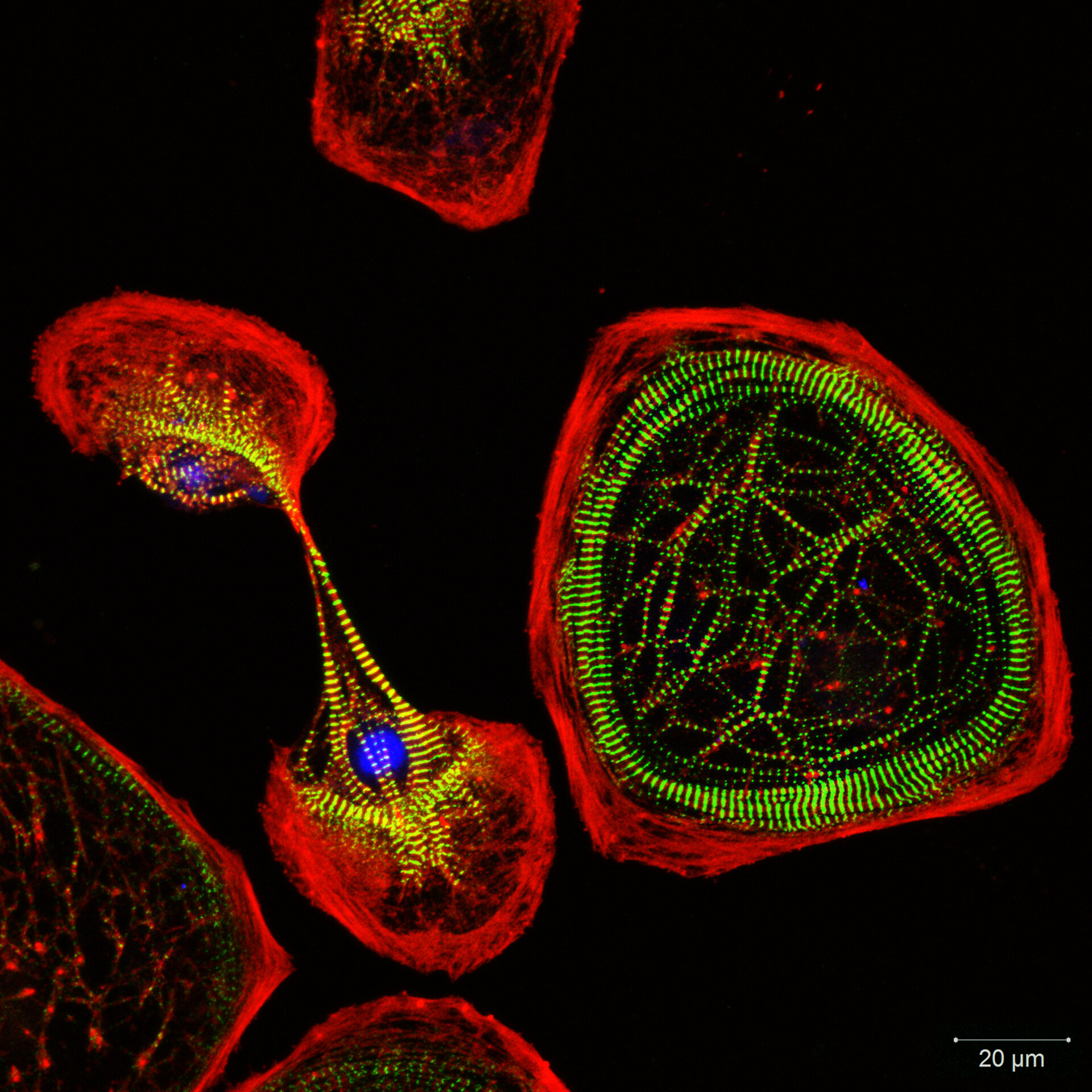
[In this image] Myofibrils in cultured cardiomyocytes.
Photo source: https://christianz.artstation.com/
Physiology of cardiomyocytes – the mechanism of cardiac contraction
Cardiomyocytes go through a contraction-relaxation cycle that enables cardiac muscles to pump blood throughout the body. This is achieved through a process known as excitation-contraction coupling (ECC) that converts action potential (an electric stimulus) into muscle contraction.
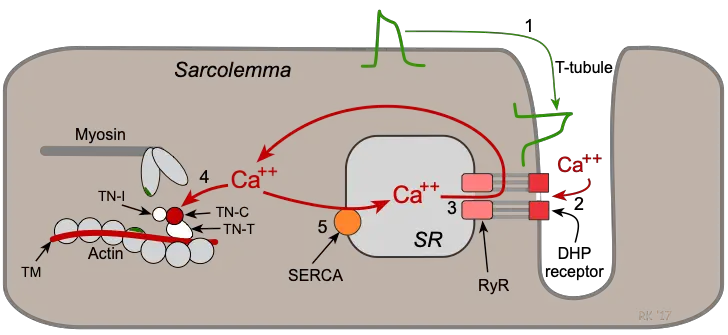
[In this figure] Schematic diagram of the process of cardiac excitation-contraction coupling.
Key steps in the cardiac excitation-contraction coupling:
Step 1: An action potential is induced by pacemaker cells. It travels along the sarcolemma and down into the T-tubule system to depolarize the cell membrane.
Step 2: Calcium channels in the T-tubules are activated by the action potential and permit calcium entry into the cell.
Step 3: Calcium influx triggers a subsequent release of calcium that is stored in the sarcoplasmic reticulum (SR).
Step 4: Free calcium binds troponin-C (TN-C) that is part of the regulatory complex attached to the thin filaments. Calcium binding moves the troponin complex from the actin binding site. As a result, actin is free to bind myosin. The actin and myosin filaments slide past each other thereby shortening the sarcomere length, thus initiating contraction.
Step 5: At the end of a contraction, calcium entry into the cell slows and calcium is sequestered by the SR by calcium pumps. Lowering the cytosolic calcium concentration releases myosin-actin binding and the initial sarcomere length is restored.
Cardiomyocytes and human heart development
In human beings (and many other animals), cardiomyocytes are the first cells to terminally differentiate, thus making the heart one of the first organs to form in a developing fetus. This makes sense because the function of the circulatory system is so crucial for a growing embryo so that the heart is the top priority.
In the embryo of a mouse, for instance, precursor cells of the cardiac muscles have been shown to start developing about 6 days after fertilization. In human embryos, the heart begins to beat at about 22-23 days, with blood flow beginning in the 4th week. The heart is therefore one of the earliest differentiating and functioning organs.
The heart forms initially in the embryonic disc as a simple paired tube (heart tube formation; week 3) derived from mesoderm. Then, the heart tubes loop and begin segmenting to separate chambers primitive atrium, and primitive ventricle. During this period, the first heartbeat begins.
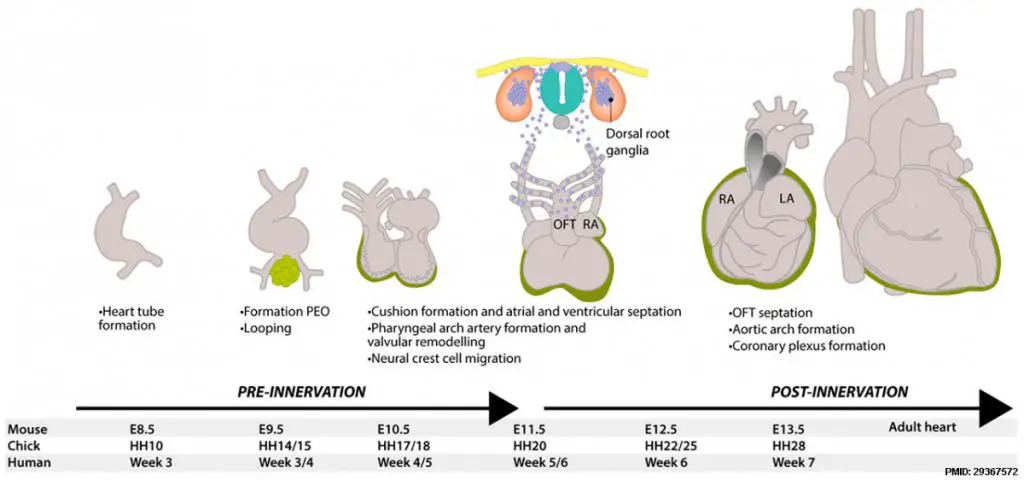
[In this figure] The timeline of heart development.
LA means left atrium; RA means right atrium. For more details, see
https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Heart_Development
Here, cardiomyocytes grow into a spongy-like tissue (cardiac jelly), called trabeculation, to build up the thickness of myocardial muscles. Thus, the heart begins to resemble the adult heart in that it has two atria, two ventricles, and the aorta forming a connection with the left ventricle while the pulmonary trunk forms a connection with the right ventricle.
As you can see that our hearts went through a complex developmental process. Inevitably, heart developmental abnormalities could happen (affect 8-10 of every 1000 births in the United States).
Cardiomyocytes renewal
Can cardiomyocytes divide? Scientists used to believe that damaged human cardiac muscles cannot regenerate themselves by cell division in adults. In other words, all cardiomyocytes are terminally differentiated. In humans, our cardiomyocytes lose the ability to divide at around 7 days after birth. However, studies have recently shown that myocytes renew at a significantly low rate throughout the life of an individual. For instance, for younger people, about 25 years of age, the annual turnover of cardiomyocytes is about 1 percent. This, however, decreases to about 0.45 percent for older individuals (75 and above). Over the lifespan of an individual, less than 50 percent of these cells are renewed. Comparing to many of the other cells, cardiomyocytes have a very long lifespan. In contrast, small intestine epithelium renews every 2-7 days and hepatocytes (liver cells) renew every 0.5-1 year.

[In this figure] Radiocarbon dating establishes the age of human cardiomyocytes.
Scientists used a pretty smart way to estimate the turnover of human heart cells. Generally speaking, the half-life of 14C is too long to date a lifetime of less than a century. However, the dramatic increase in the atmospheric 14C caused by nuclear bomb tests (during the Cool War) in the 1950s and 1960s increased the sensitivity of radiocarbon dating to a temporal resolution of 1-2 years.
Photo source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5837331/
Cardiac repair and cardiomyocyte regeneration
Low turnover of human cardiomyocytes suggests that the regenerative ability of cardiac muscles may be poor (another example is neural cells in the brain). In the event of injuries or myocardial infarction, the injured heart muscles of human beings do not regenerate sufficiently to allow the heart to heal itself. Instead, fibrotic scar tissue forms in the injured site (fibrosis), and the heart functions are compromised, leading to heart failure.
Currently, a number of methods have been studied to repair a broken heart by regenerating cardiomyocytes. These new inventions benefit from the recent advances in biotechnology, especially stem cell biology, regenerative medicine, and tissue engineering. Hopefully, this can bring new therapeutic options to patients with cardiovascular diseases in the near future.
(1) Adult cardiac stem (progenitor) cells
Studies suggested that even in adults, a very small population of progenitor cells reside in the heart and are capable of producing new cardiac myocytes. These cells, known as cardiac stem cells, may not be able to regenerate fast enough to repair a large area of damaged myocardium naturally in humans. However, these cells have shown to be powerful in regenerative capability in other species, like zebrafish.
Scientists believe that once we understand these cardiac progenitors more, we may isolate and expand these cells in quantity, and transplant them to repair damaged heart tissues. For example, we already learned that these cardiac stem cells express cell surface markers like c-Kit (sca-1 in mouse) and aggregate into cardiac spheres.
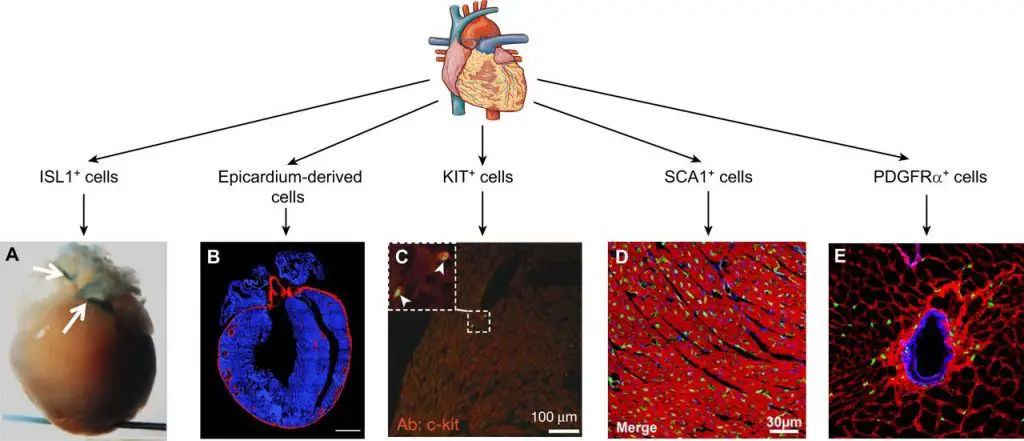
[In this figure] Multiple different stem cell populations have been described in the adult heart, including c-Kit and Sca-1 cells that were shown to be cardiac progenitors.
Photo source: https://dev.biologists.org/content/143/8/1242
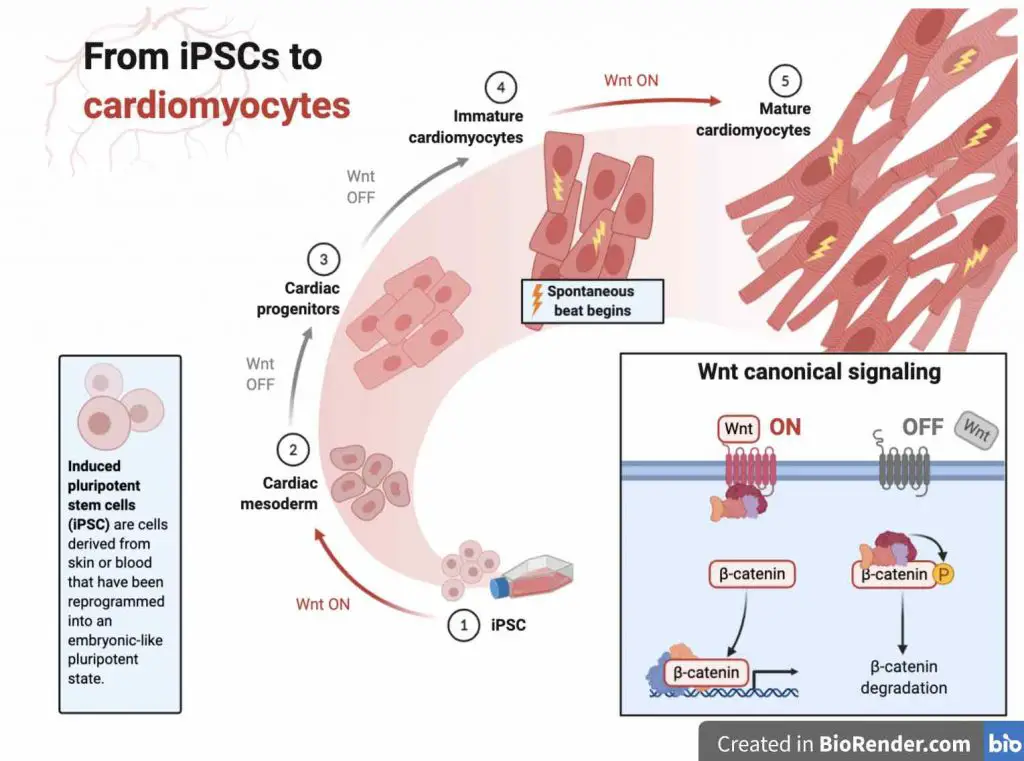
(2) iPSC-derived cardiomyocytes
Induced pluripotent stem cell (iPSC) technology is a huge revolution in biotechnology. Patient’s cells (easily obtained from skin biopsy or even urine) can be converted into powerful pluripotent stem cells that have unlimited proliferation capacity and can differentiate any cell type of our body. This eliminates the need to use human embryos for this purpose. Furthermore, these cells are autologous, meaning they won’t be rejected by the immune system after transplantation.
Using iPSC technology, researchers have been able to obtain unlimited amounts of functional cardiomyocytes for cell transplantation. Basically, they control the Wnt pathway to convert iPSCs to mesodermal progenitor cells, then play with several growth factors to direct the cardiac vascular progenitors (Flk1+). Following glucose starvation, pure cardiomyocytes can be selected. You can even see these cells beating in the dish.
Therapeutic implantation of iPSC-derived cardiomyocytes progresses pretty fast. We already witnessed successful cell engraftment and cardiac repairing in non-human primates and human patients.
[In this video] Heart cells derived from iPSC stem cells beating in a cell culture dish.
(3) Direct reprogramming of cardiac fibroblasts
Cardiac fibroblasts make up a significant portion of the total cardiac cells. In the injured heart, these fibroblasts will become active myofibroblasts and form scar tissue. Myofibroblasts survive very well and have ability to coupled with neighboring cells; therefore, myofibroblasts have been shown to be particularly ideal for direct reprogramming to convert them into cells that resemble cardiomyocytes.
Over the past decade, a number of studies have been successfully conducted, reprogramming fibroblasts into cardiomyocyte-like cells. In principle, scientists expressed transcription factors (i.e., Gata4, Mef2c, and Tbx5) that play critical roles in cardiomyocyte differentiation to force the conversion of fibroblasts. Ideally, these genes can be delivered directly to the injured heart via viruses or nanoparticles to perform “in situ” reprogramming.
(4) Cell cycle re-entry of mature cardiomyocytes
Scientists also put their efforts into how to stimulate mature cardiomyocytes to proliferate again (Mature cardiomyocytes typically do not proliferate.) This strategy, called cell cycle re-entry, recently gained success by screening many cell-cycle regulators. Scientists found a combination of cyclin-dependent kinases (CDK) and cyclins, or regulators of the Hippo-YAP signaling pathway can do so. These findings reveal the possibility to efficiently unlock the proliferative potential in cells that had terminally exited the cell cycle.
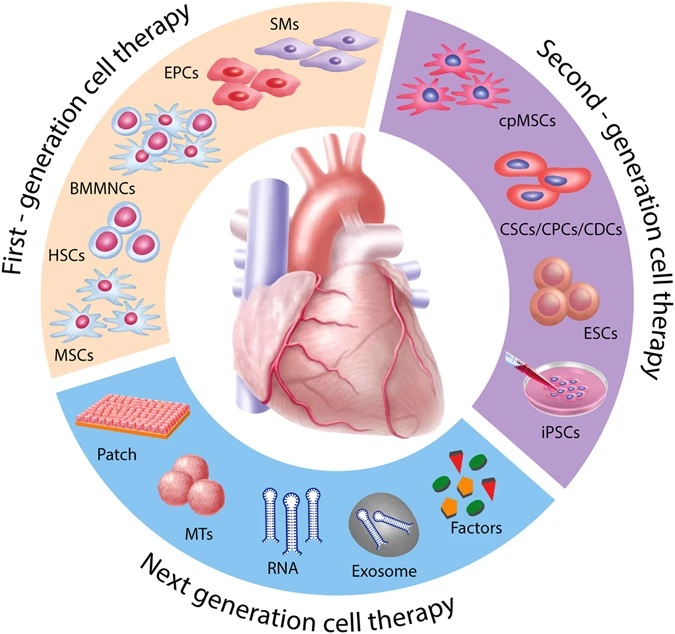
[In this figure] Potential cardiac regenerative therapies.
Photo source: https://www.nature.com/articles/s41536-017-0024-1
Microscopy and histology to research cardiomyocytes
Cardiomyocytes can be observed by staining of histological sections of the heart. Since the heart is a 3-D organ, make sure you cut the heart at the right angle.
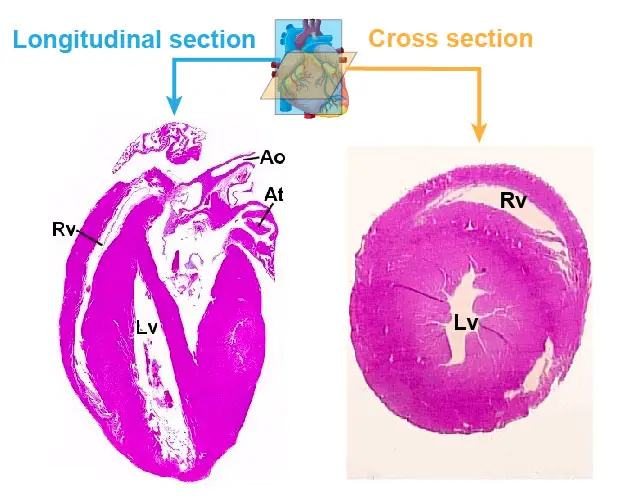
[In this figure] (Left) A longitudinal section through both ventricles should be made from the base to the apex of the heart. (Right) A cross-section of the heart. H&E staining.
(Ao: aorta, At: atrium, Lv: left ventricle, Rv: right ventricle)
Common histological staining for heart tissues includes Hematoxylin and eosin (H&E) and Masson’s trichrome staining.
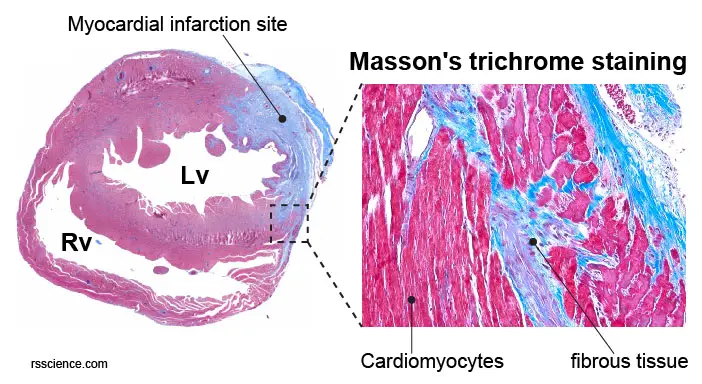
[In this figure] A cross section of mouse heart stained by Masson’s trichrome. Blue color indicates the formation of fibrous scar tissues in the infarction area.
Cardiomyocytes can be isolated from cardiac tissue by enzymatic digestion and cultured for a short period of time. Cardiomyocytes can also be derived from iPSCs following well-established protocols. Once the cells have been fixed and permeabilized on the slide or dish, then they are ready for staining and viewing. Below is a list of commonly used cardiac cell markers for immunofluorescence or immunohistochemistry staining.
Calcium Imaging of cardiomyocytes is routinely done by calcium trackers (like Fura-2AM) in cultured cardiomyocytes.
Cell markers for cardiomyocytes
Surface and structural cardiac markers:
- Troponin T
- MYH6
- MYH7
- Myosin Heavy Chain
- Connexin 37/GJA4
- Connexin 40/GJA5
- Sarcomeric α-Actinin
Cardiac-specific Transcriptional factors:
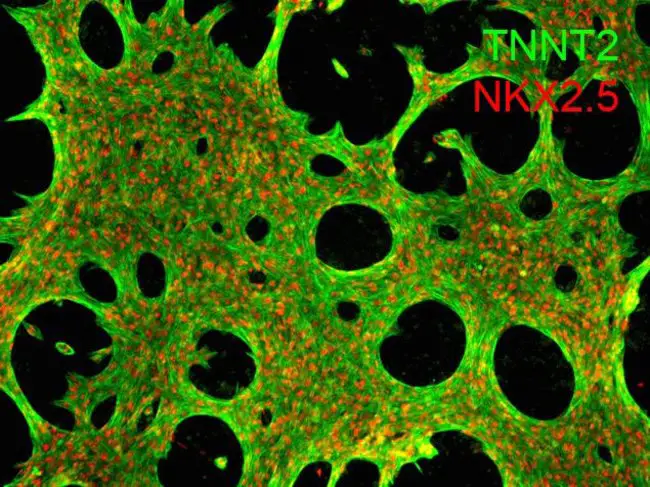
[In this figure] Immunofluorescent staining of human iPSC-derived cardiomyocytes using both cytoskeleton markers (TNNT2 or cTNT) and transcriptional factors (NKX2.5).
Photo source: https://www.thermofisher.com/order/catalog/product/A25973#/A25973
Other terms related to cardiomyocytes physiology
- Hypertrophy – Increases in cardiac myocyte size
- Cardiomyopathy – Diseases characterized by disruptions to cardiac muscle cell growth or organization. Cardiomyopathy can be caused by genetic, endocrine, environmental, or other factors.
- Myocytolysis – Significant damage to cardiac muscle cells.
References
“Cardiomyocytes – a general description, the intercalated discs, the sarcomere, T-tubules and cardiac mitochondria.”
“Cell Communications in the Heart” by Daniela Tirziu, Frank J. Giordano, and Michael Simons. Circulation. 2010 Aug 31; 122(9): 928–937.
“Cardiovascular System – Heart Development” by Mark Hill
“Desmosomes” by Adi D. Dubash and Kathleen J. Green
“How quickly do different cells in the body replace themselves?”
“Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors” Cell. 2010 Aug 6; 142(3): 375–386.
“Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration” Cell. 2018 Mar 22; 173(1): 104–116.e12.
“Hippo pathway effector Yap promotes cardiac regeneration” PNAS. 2013 Aug 20;110(34):13839-44.
“Medical Physiology, 3rd Edition: Cardiac Muscle”
“Cardiac Muscle Tissue” Lumen Anatomy and Physiology I
“Cardiomyocyte renewal in the human heart: insights from the fall-out”
“Cardiomyocyte Markers”
“Developmental origin and lineage plasticity of endogenous cardiac stem cells”
“Actin, Myosin, and Cell Movement” The Cell: A Molecular Approach. 2nd edition.
“Cardiovascular Physiology Concepts” by Richard E. Klabunde